Team:NTNU Trondheim/Project
From 2012.igem.org
| Line 1: | Line 1: | ||
{{:Team:NTNU_Trondheim/Templates/Header}} | {{:Team:NTNU_Trondheim/Templates/Header}} | ||
| + | <html> | ||
| + | <div class="container"> | ||
| + | <div class="page-header-top"> | ||
| + | <h1>Project description <small>Bacterial Anti-Cancer Kamikaze explained</small></h1> | ||
| + | </div> | ||
| + | </div> | ||
| - | |||
| + | <div class="container main-container"> | ||
| + | </html> | ||
__TOC__ | __TOC__ | ||
| Line 15: | Line 22: | ||
Differentiated cells (“normal” cells) can produce high amount of lactate in an anaerobic environment, but this is not a normal environment for the cells in our body. Under normal circumstances, the differentiated cells will primarily metabolize glucose to carbon dioxide in the citric acid cycle (TCA), and have a very low production of lactate. Cancer cells behave differently. They produce large amounts of lactate regardless of the oxygen concentration in their surroundings [http://www.ncbi.nlm.nih.gov/pubmed/19460998], [http://cancerres.aacrjournals.org/content/58/7/1408.full.pdf+html]. The reason that we want to use oxygen as a trigger factor is that tumors exists in an environment deprived of oxygen [http://www.springerlink.com/content/a60537w7085574v4/]. | Differentiated cells (“normal” cells) can produce high amount of lactate in an anaerobic environment, but this is not a normal environment for the cells in our body. Under normal circumstances, the differentiated cells will primarily metabolize glucose to carbon dioxide in the citric acid cycle (TCA), and have a very low production of lactate. Cancer cells behave differently. They produce large amounts of lactate regardless of the oxygen concentration in their surroundings [http://www.ncbi.nlm.nih.gov/pubmed/19460998], [http://cancerres.aacrjournals.org/content/58/7/1408.full.pdf+html]. The reason that we want to use oxygen as a trigger factor is that tumors exists in an environment deprived of oxygen [http://www.springerlink.com/content/a60537w7085574v4/]. | ||
| - | ==Project | + | ==Project overview== |
Our goal for this years competition is to create a genetic circuit which enables ''E. coli'' cells to detect and attack cancer cells. To do this, the cells should produce a tumor inhibitor or toxin effective at killing cancer cells, and respond to environmental cues indicating the presence of cancer cells by undergoing [http://en.wikipedia.org/wiki/Lysis lysis], releasing the anti-cancer agent into the surrounding environment. In principle, bacteria could be used in "search and destroy" missions against cancer inside the human body. We wish to develop our genetic circuit as a proof-of-concept of one such strategy. | Our goal for this years competition is to create a genetic circuit which enables ''E. coli'' cells to detect and attack cancer cells. To do this, the cells should produce a tumor inhibitor or toxin effective at killing cancer cells, and respond to environmental cues indicating the presence of cancer cells by undergoing [http://en.wikipedia.org/wiki/Lysis lysis], releasing the anti-cancer agent into the surrounding environment. In principle, bacteria could be used in "search and destroy" missions against cancer inside the human body. We wish to develop our genetic circuit as a proof-of-concept of one such strategy. | ||
| Line 21: | Line 28: | ||
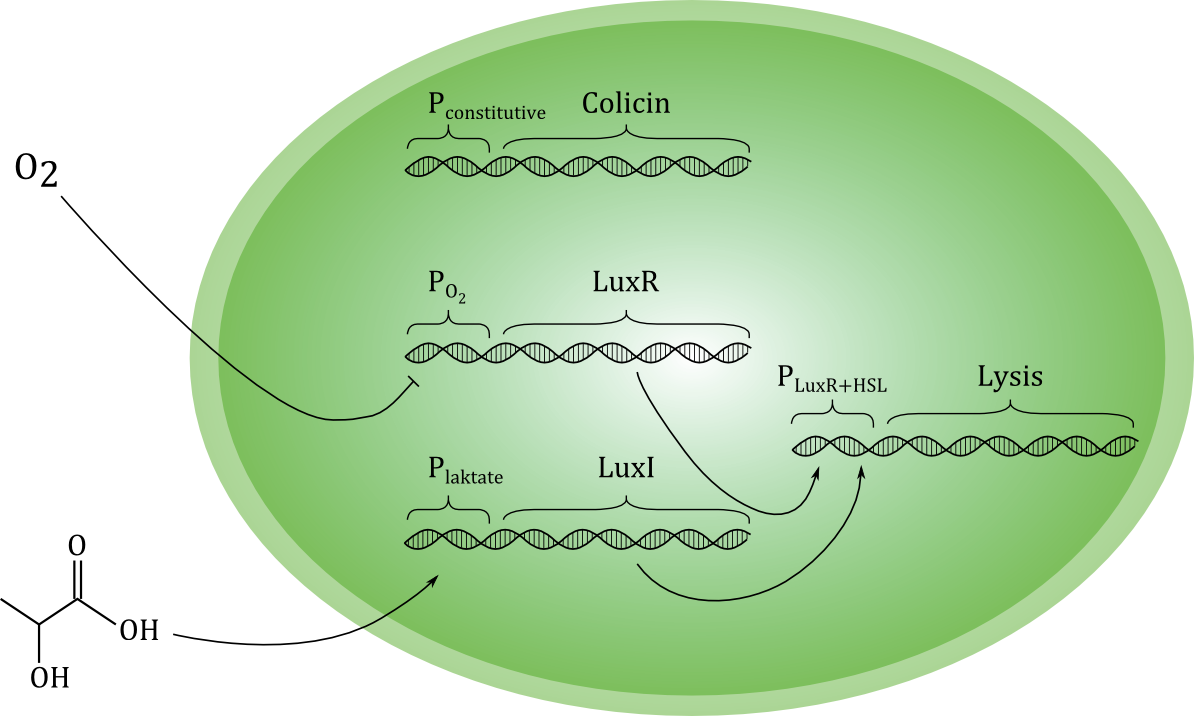
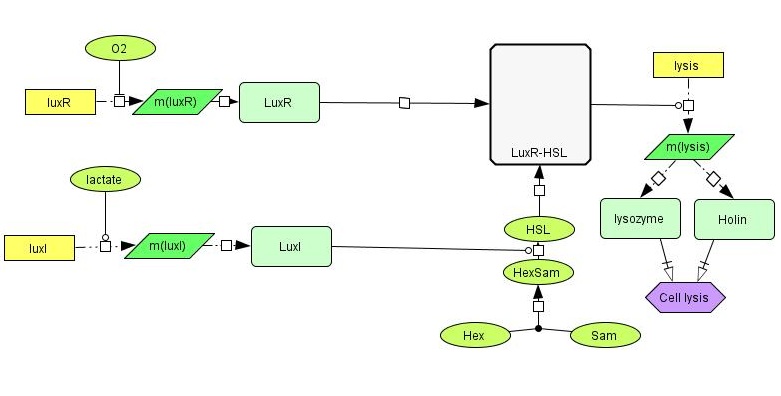
A sketch of our current planned design for the circuit is shown below. | A sketch of our current planned design for the circuit is shown below. | ||
| - | [[File:Grønn_krets.png|center| | + | [[File:Grønn_krets.png|thumb|center|600px|A sketch of our planned genetic circuit]] |
Ideally, the toxin should only be released if cancer cells are actually encountered, and never otherwise. To achieve this, the lysis-inducing part of the circuit should be regulated such that it is highly unlikely to activated in other situations. One possible way is to use a signal molecule that is solely associated with cancer cells to activate the lysis device. Another possibility is to use a combination of environmental cues that together indicate a high likelihood of cancer presence. We have chosen the latter strategy, and the environmental cues we aim to use are low O2 and high lactate concentrations. | Ideally, the toxin should only be released if cancer cells are actually encountered, and never otherwise. To achieve this, the lysis-inducing part of the circuit should be regulated such that it is highly unlikely to activated in other situations. One possible way is to use a signal molecule that is solely associated with cancer cells to activate the lysis device. Another possibility is to use a combination of environmental cues that together indicate a high likelihood of cancer presence. We have chosen the latter strategy, and the environmental cues we aim to use are low O2 and high lactate concentrations. | ||
| Line 48: | Line 55: | ||
LuxI is a acyl homoserine lactone syntase, an enzyme catalyzing the production of a homoserine lactone (HSL) from the precursors S-adenosyl methionine (Sam) and hexanoyl-ACP (Hex). The normal function of LuxI is as part of the bacterial lux system, a [http://en.wikipedia.org/wiki/Quorum_sensing quorom-sensing] system allowing cells in proximity to sense each others presence, estimate the local cell population and regulate [https://www.bio.cmu.edu/courses/03441/TermPapers/97TermPapers/lux/bioluminescence.html bioluminescence]. LuxI is available as the BioBrick part <partinfo>BBa_K092400</partinfo>. | LuxI is a acyl homoserine lactone syntase, an enzyme catalyzing the production of a homoserine lactone (HSL) from the precursors S-adenosyl methionine (Sam) and hexanoyl-ACP (Hex). The normal function of LuxI is as part of the bacterial lux system, a [http://en.wikipedia.org/wiki/Quorum_sensing quorom-sensing] system allowing cells in proximity to sense each others presence, estimate the local cell population and regulate [https://www.bio.cmu.edu/courses/03441/TermPapers/97TermPapers/lux/bioluminescence.html bioluminescence]. LuxI is available as the BioBrick part <partinfo>BBa_K092400</partinfo>. | ||
| - | [[File:NTNU_3OHSL.gif||center|[http://partsregistry.org/3OC6HSL 3-oxohexanoyl-homoserine lactone] is synthesized by LuxI]] | + | [[File:NTNU_3OHSL.gif|thumb|center|400px|[http://partsregistry.org/3OC6HSL 3-oxohexanoyl-homoserine lactone] is synthesized by LuxI]] |
LuxR is another protein in the lux system, and acts by binding to 3OC6HSL, the product formed by the catalytic activity of LuxI. LuxR-HSL then acts on gene promoters to regulate genetic expression. We will use the simultaneous expression of LuxR and LuxI leading to the the formation of LuxR-HSL to activate the lysis device which causes the cell to dissolve and release its produced toxin. | LuxR is another protein in the lux system, and acts by binding to 3OC6HSL, the product formed by the catalytic activity of LuxI. LuxR-HSL then acts on gene promoters to regulate genetic expression. We will use the simultaneous expression of LuxR and LuxI leading to the the formation of LuxR-HSL to activate the lysis device which causes the cell to dissolve and release its produced toxin. | ||
| Line 54: | Line 61: | ||
The lysis device was made by the [https://2008.igem.org/Team:UC_Berkeley UC Berkeley iGEM 2008] team. The genes are from the [http://en.wikipedia.org/wiki/Enterobacteria_phage_T4 bacteriophage T4] (a virus that infects bacteria). We will use it as-is, placing a self-chosen promoter in front of the part to control the initiation of cell lysis. | The lysis device was made by the [https://2008.igem.org/Team:UC_Berkeley UC Berkeley iGEM 2008] team. The genes are from the [http://en.wikipedia.org/wiki/Enterobacteria_phage_T4 bacteriophage T4] (a virus that infects bacteria). We will use it as-is, placing a self-chosen promoter in front of the part to control the initiation of cell lysis. | ||
| - | [[ | + | [[File:S-Adenosyl methionine.png|thumb|center|300px|S-Adenoysyl-methionine (SAM)]] |
| + | <!--The structure of Hexanoyl-ACP: | ||
| + | [[file:HexanoylACP_structure.jpg|center|300px]] (from the RCSB Protein Databank, entry [http://www.rcsb.org/pdb/explore.do?structureId=2FAC 2FAC])--> | ||
| - | + | <!--==References== | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | ==References== | + | |
Lactate sensitive promoter: | Lactate sensitive promoter: | ||
| Line 91: | Line 87: | ||
Lysis device: | Lysis device: | ||
| - | [https://2008.igem.org/Team:UC_Berkeley/LysisDevice UC Berkeley iGEM 2008: Lysis Device] | + | [https://2008.igem.org/Team:UC_Berkeley/LysisDevice UC Berkeley iGEM 2008: Lysis Device]--> |
Revision as of 17:04, 25 September 2012
 "
"