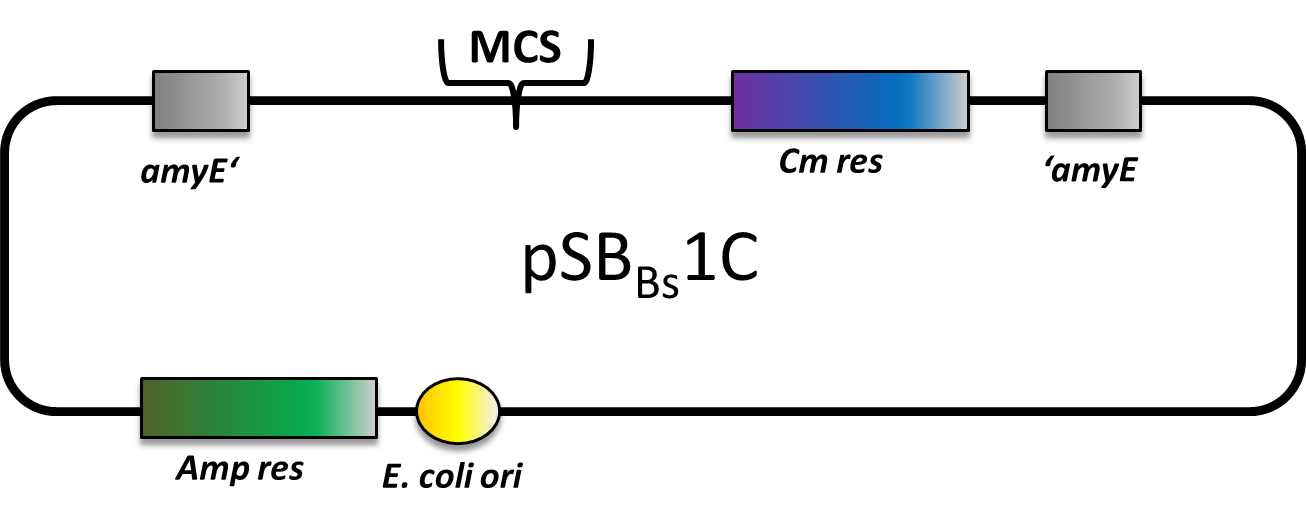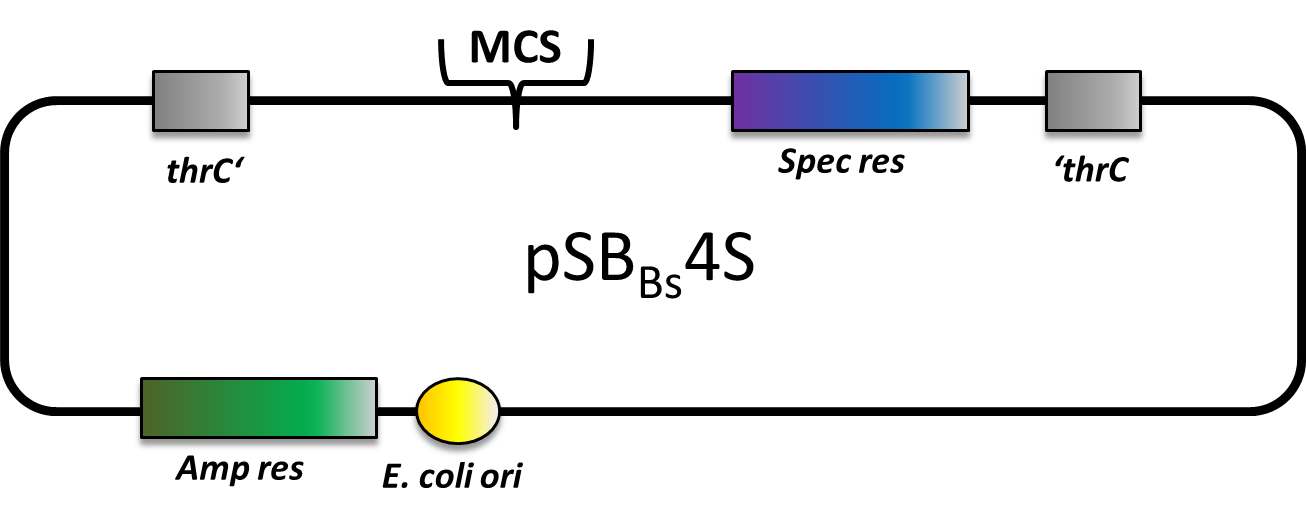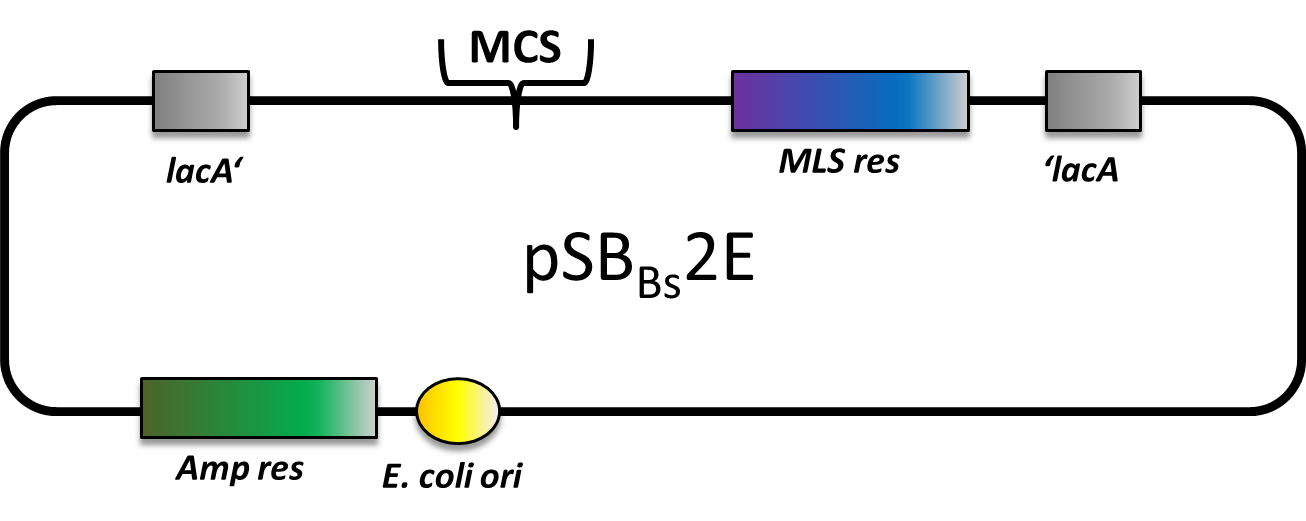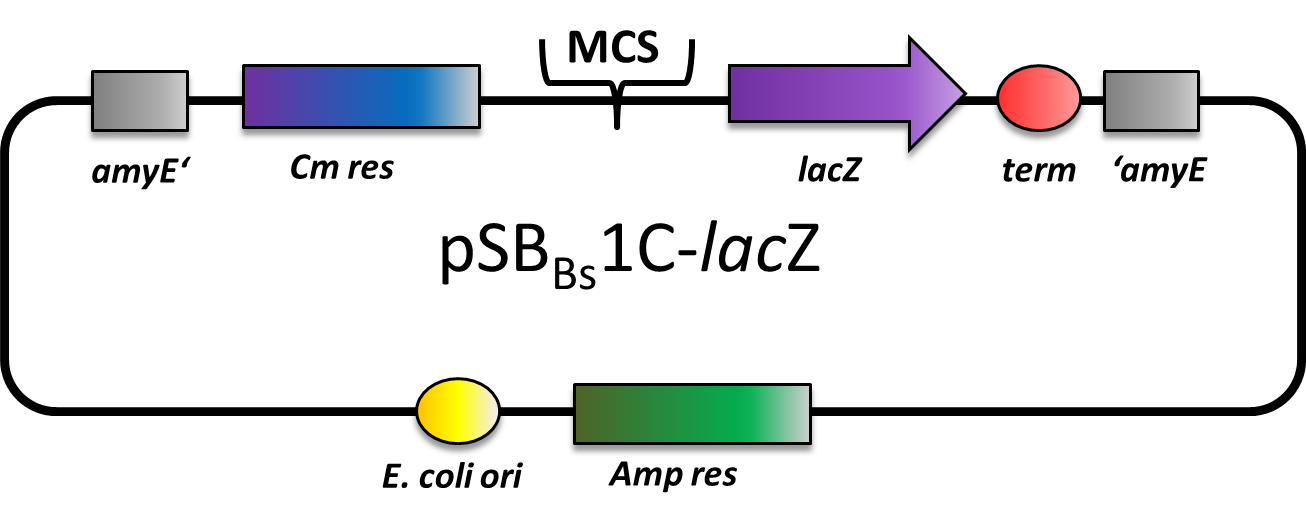Team:LMU-Munich/Data/Vectors
From 2012.igem.org
| Line 1: | Line 1: | ||
{{:Team:LMU-Munich/Templates/Page Header|File:Team-LMU_culture_tubes.resized.jpg|3}} | {{:Team:LMU-Munich/Templates/Page Header|File:Team-LMU_culture_tubes.resized.jpg|3}} | ||
| + | |||
| + | |||
| + | [[Image:BacillusBioBrickBox.png|100px|right|link=Team:LMU-Munich/Team:LMU-Munich/Bacillus_BioBricks]] | ||
| + | |||
| + | |||
==''Bacillus subtilis'' vectors== | ==''Bacillus subtilis'' vectors== | ||
Revision as of 15:30, 25 September 2012

The LMU-Munich team is exuberantly happy about the great success at the World Championship Jamboree in Boston. Our project Beadzillus finished 4th and won the prize for the "Best Wiki" (with Slovenia) and "Best New Application Project".
[ more news ]

Bacillus subtilis vectors
For the detailed protocols, please see our protocol section.
Overview
| Name BioBrick | E. coli res. | B. subt. res. | Insertion locus | Description | Cloning status | Works? |
|---|---|---|---|---|---|---|
| pSBBs1C [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823023 (BBa_K823023)] | Amp | Cm | amyE | empty | ||
| pSBBs4S [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823022 (BBa_K823022)] | Amp | Spec | thrC | empty | ||
| pSBBs2E [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823027 (BBa_K823027)] | Amp | MLS | lacA | empty | in work | not tested |
| pSBBs1C-lacZ [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823021 (BBa_K823021) ] | Amp | Cm | amyE | lacZ reporter | ||
| pSBBs3C-luxABCDE [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823025 (BBa_K823025)] | Amp | Cm | sacA | luxABCDE reporter | ||
| pSBBs4S-PXyl [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823024 (BBa_K823024)] | Amp | Spec | thrC | Xylose-promoter | refuses transformation | |
| pSBBs0K-Pspac [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823026 (BBa_K823026)] | Amp | Kan | replicative | IPTG-promoter | unexpected | |
| Sporovector [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823026 (BBa_K823054)] | Amp | Spec | thrC | to make Sporobeads | not tested |
pSBBs1C
pSBBs1C is one of our empty vectors. It integrates into the amyE locus of Bacillus subtilis and carries a Chloramphenicol resistance as well as an Ampicillin resistance for E. coli. Inbetween the two recombination sites, there is the constitutively expressed Cm resistance and the multiple cloning site which contains RFP to simplify the insertion of your BioBrick.
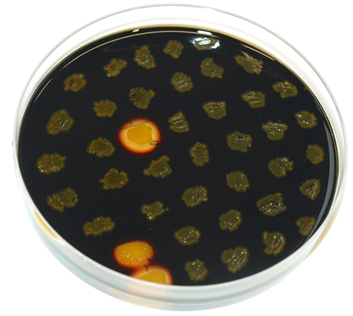
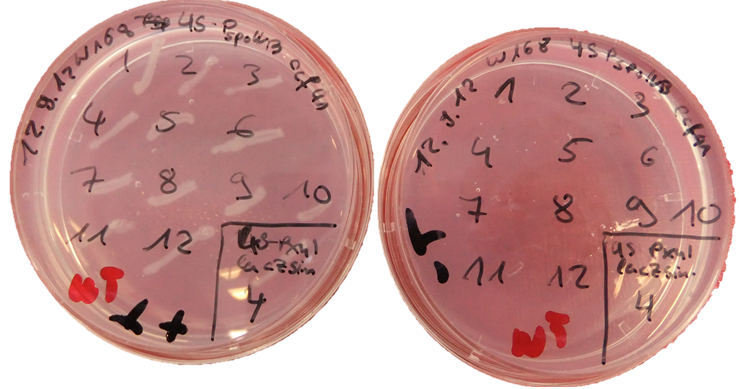
The plasmid is then transformed into B. subtilis and checked via the resistance for "integration at all" and via a starch test for "integration into the right locus". Please note that the colonies without a bright surounding are correct.
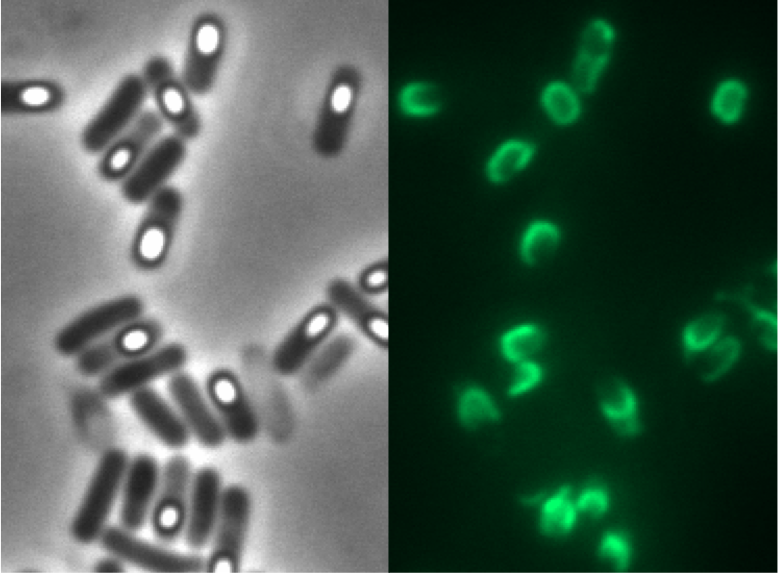
We tested this vector with various inserts, for example the final construct of our Sporobeads with GFP. The starch test shows that the insertion in in the amyE locus and fluorescence microscopy reveals the functionality of the construct.
pSBBs4S
pSBBs4S also is an empty vector with only the multiple cloning site and the spectinomycine resistence gene in between the homologous regions. This vector integrates into the thrC locus of B. subtilis.
We tested the integration of this plasmid with the threonine test and a construct from the Suicide switch as insert.
pSBBs2E
pSBBs2E is our third empty vector, aiming to provide a set of three empty vectors with three different integration loci and three different resistances for full flexibility in their combinations.
This vector is still in work. By now, only the multiple cloning site is missing. Of course, it is not tested yet.
pSBBs1C-lacZ
pSBBs1C-lacZ is a reporter vector which includes a ribosome binding site, a β-galactosidase (lacZ) and a terminator downstream of the multiple cloning site. Also, a chloramphenicol resistance is in between the two flanking homolgy regions. This vector integrates into the amyE locus which is tested by the starch test.
This vector was used for several promoter evaluations.
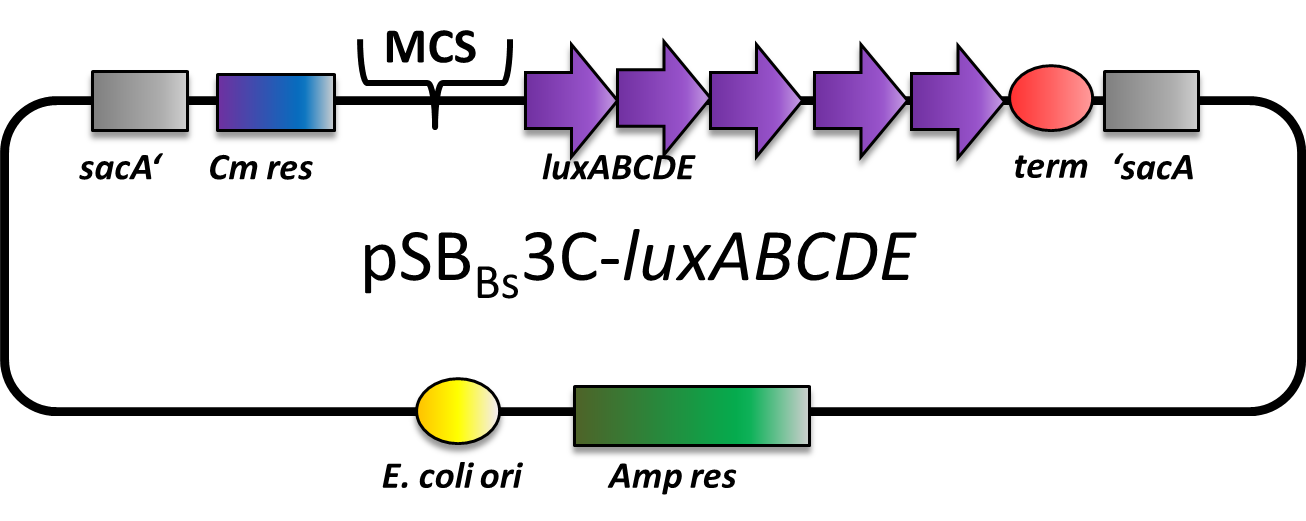
pSBBs3C-luxABCDE
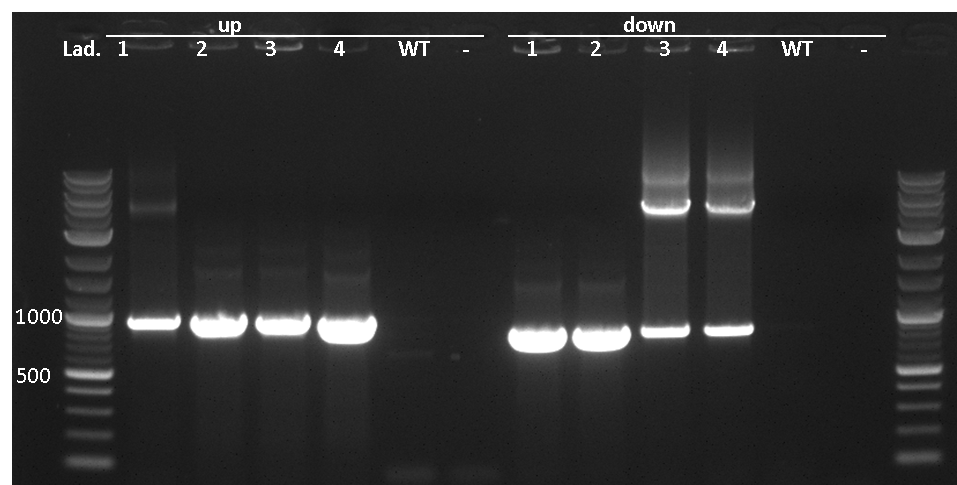
pSBBs3C-luxABCDE is our second reporter vector. Downstream of the multiple cloning site, there are a ribosome binding site, the luxABCDE operon and a terminator. Also, a chloramphenicol resistance is in between the two flanking homolgy regions. This vector integrates into the sacA locus of B. subtilis which is tested for by colony PCR.
One primer is located outside of the region and its respective partner is located on the inegrative part of the vector, facing outwards. gDNA from W168 as a negative control should not give a PCR product.
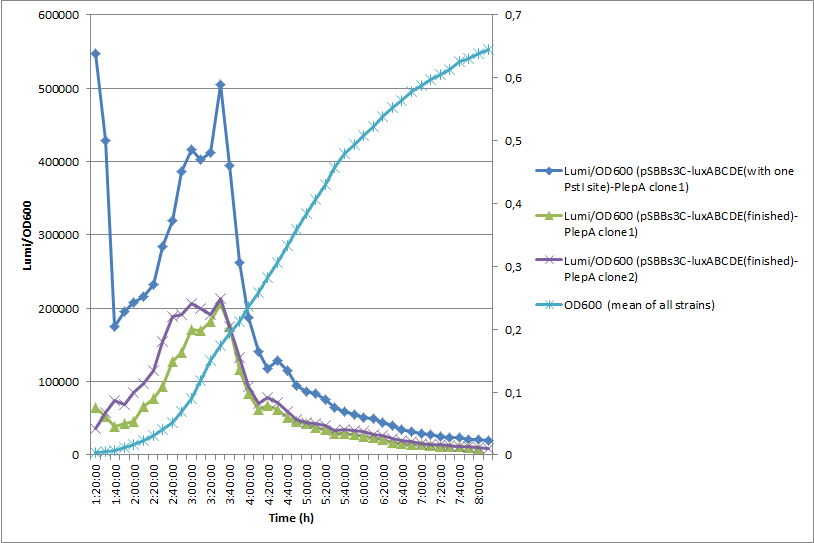
In this experiment with the same promoter PlepA the finished vector showed about half of the activity of the pre version where there was still one forbidden PstI site left.</p>
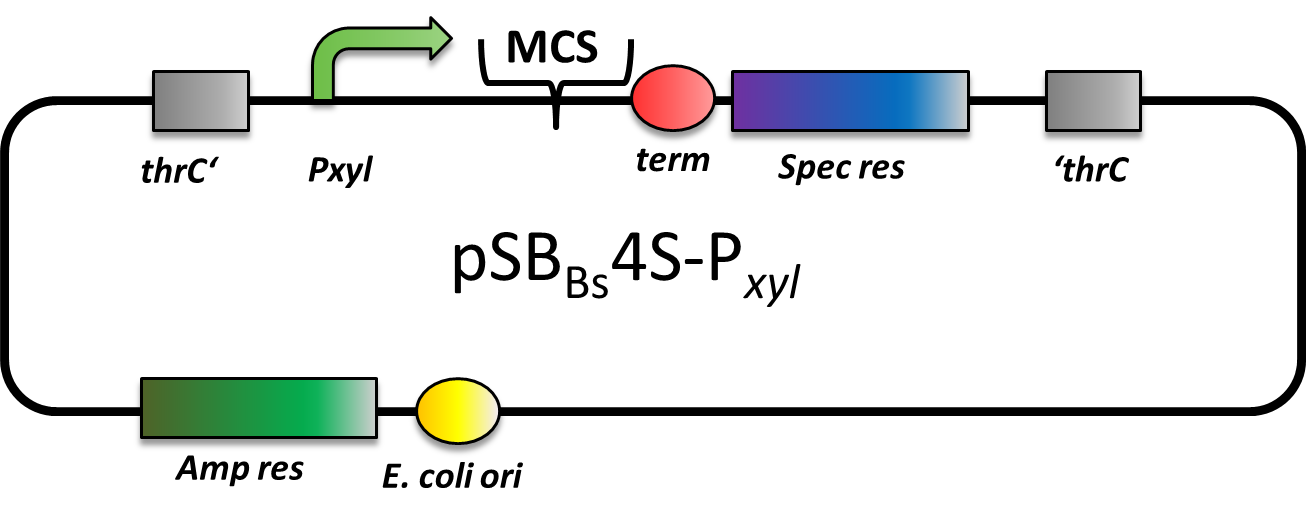
pSBBs4S-PXyl
CAUTION: DOES NOT WORK! CANNOT BE TRANSFORMED INTO B. SUBTILIS
pSBBs4S-PXyl is an integrative expression vector. Upstream of the multiple cloning site, there is PXyl, a xylose-inducible promoter, and downstream there is a terminator. In absence of Xylose, PXyl is blocked by the repressor XylR which is expressed in B. subtilis and not part of this vector. Apart from that, also a spectinomycine resistance is located in between the two recombination sites. This vector integrates into the thrC locus which is checked for by the threonine test.
However, we could not manage to transform this vector into B. subtilis, although we tried three times. Also the team from Groningen tested this vector, but was not able to transform it into B. subtilis, either.
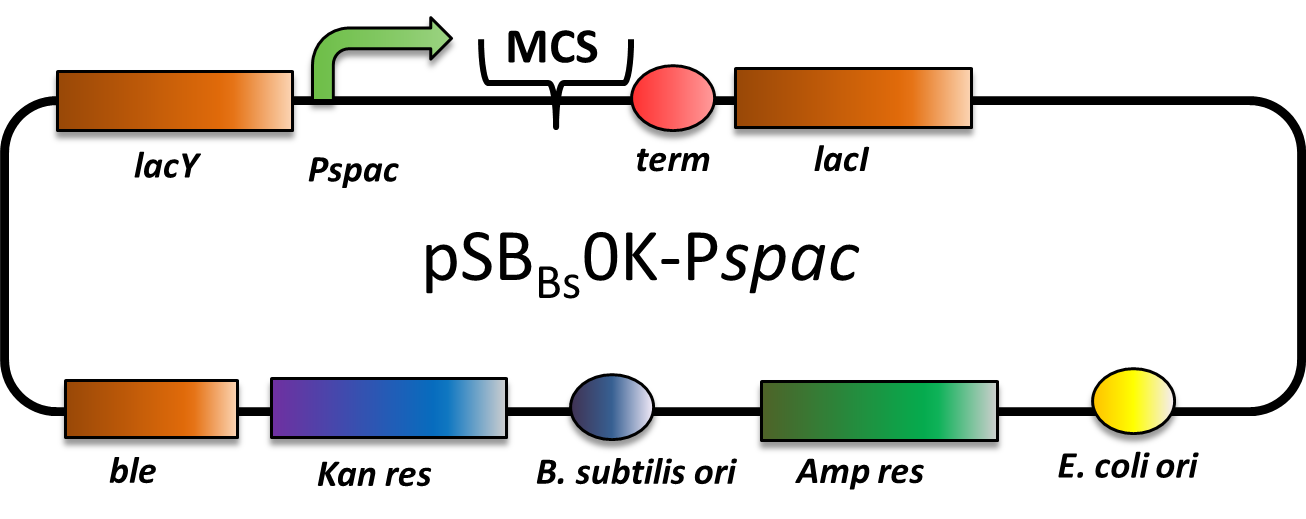
pSBBs0K-Pspac
CAUTION: THIS VECTOR DOES NOT WORK AS EXPECTED! THE PROMOTER IS STRONG CONSTITUTIVE INSTEAD OF IPTG-INDUCIBLE!
pSBBs0K-Pspac is a replicative expression vector with a kanamycin resistance. The IPTG (isopropylbeta-D-thiogalactopyranoside)-inducible Promoter Pspac is followed by the multiple cloning site and a terminator. Also expressed are lacY, a transporter for IPTG (naturally allolactose), and lac, the repressor. In presence of IPTG, LacI releases from the promoter and the gene of interest is expressed.
The ble gene encodes the bleomycin resisitance protein (BRP) which can be selected for by bleomycine or phleomycin in E. coli and B. subtilis. We did not use this resistance.
The presence of the vector can be checked for via colony PCR with the primers: CTACATCCAGAACAACCTCTGC and TTCGGAAGGAAATGATGACCTC. We did not perfom the colony PCR because we selected for functionality on plates with X-Gal and IPTG and we got blue colonies.
|
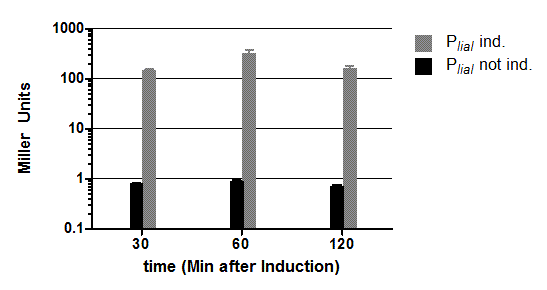
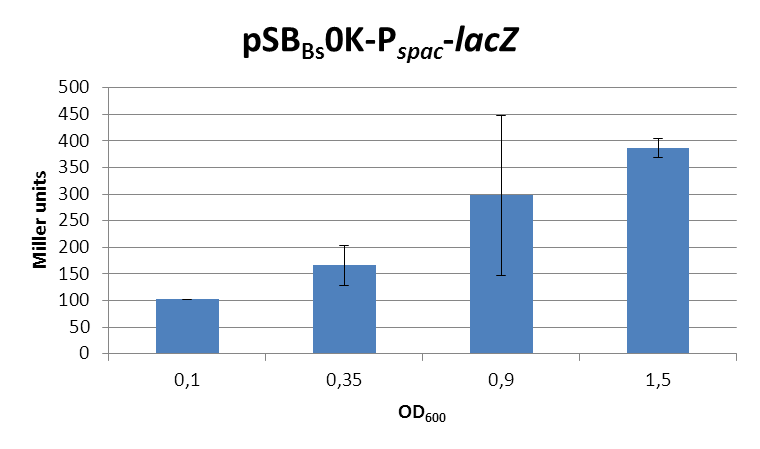
This expression vector was tested by insertion of [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823016 lacZ] into the multiple cloning site, transformation into W168 and ONPG assays. Overnight cultures were diluted 1:100 in LB-medium and incubated at 37°C, 230 rpm. Cells were harvested in 2 ml reaction tubes and frozen. For the induction assay, at an OD600 around 0.9 were split into 3 ml aliquots in test tubes with the given IPTG-concentrations and incubated for another hour. Data for splitting at OD600 around 0.3 is not shown, but similar.
|
In summary, the figures show that the expression is very strong, even without induction [in comparison to the native B. subtilis promoters] and does not change with IPTG addition. It does change depending on the growth phase, with a maximum strength in the stationary phase.
This vector was designed for overexpression of proteins, but is not inducible but constitutively active.
Sporovector
Our Sporovector is specially designed to give you the opportunity to easily create Sporobeads with any proteins you can think of. It is available as BioBrick in pSB1C3 and can be cloned in any of our empty B. subtilis vectors. We also offer it “precloned” in pSBBs4S which in this version lacks NgoMIV restriction sites.
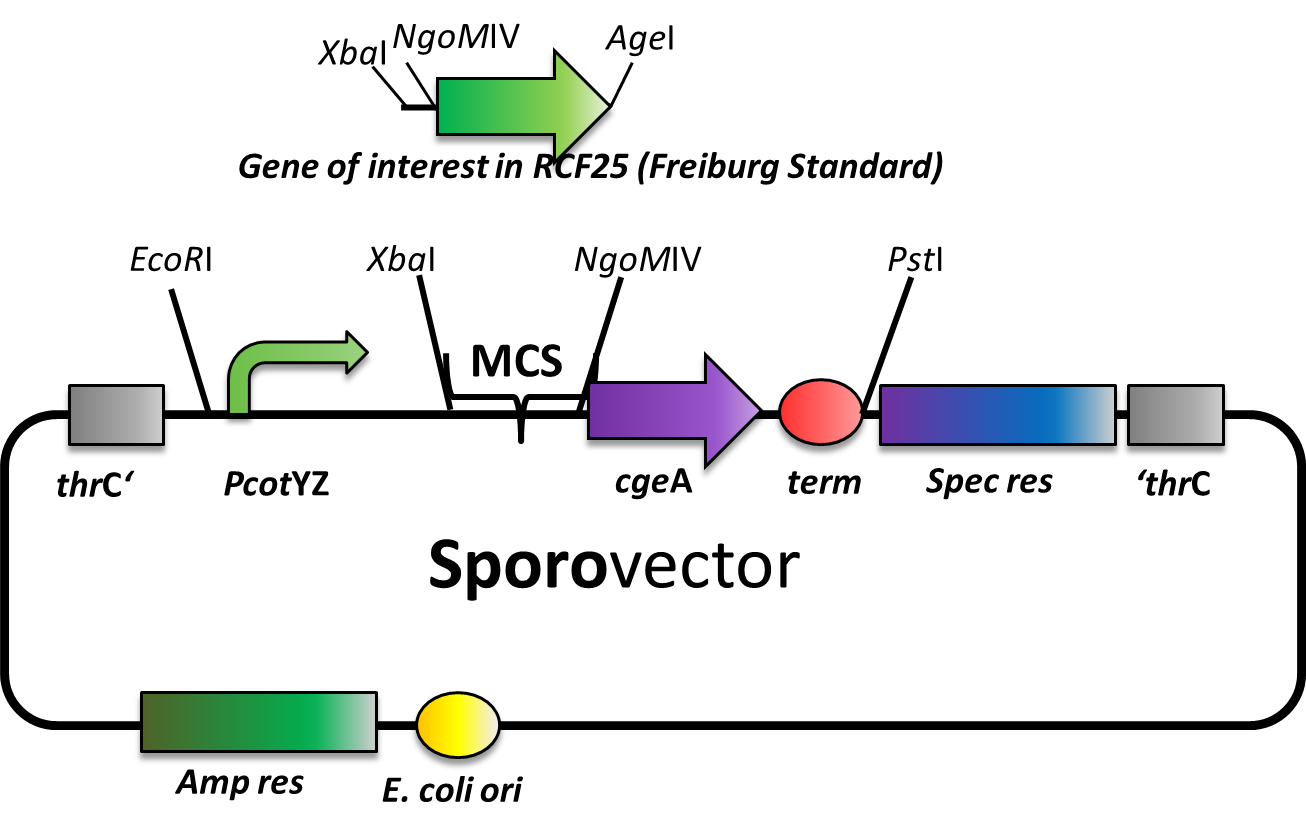 The Sporovector was created by ligation of three cut PCR-products. It is designed to allow N-terminal fusion of genes in Freiburg standard ([http://dspace.mit.edu/bitstream/handle/1721.1/45140/BBF_RFC%2025.pdf?sequence=1 RCF25]). Therefore, the gene to be inserted must be cut with XbaI and AgeI and the vector must be digested with XbaI and NgoMIV. By this digest, the RFP cassette will be removed from this vector to allow easy selection for the integration of the new insert. After ligation, there is a scar of six nucleotides between both genes but no restriction site left.
The gene fusion is controlled by the PcotYZ-promoter which was the strongest one in our promoter evaluations. The C-terminal gene part is cgeA, one of the two known spore crust proteins.
The vector is cloned but has not been tested with an insert, yet.
We are working on further Sporovectors to offer also fusion with cotZ as well as C-terminal fusions.
The Sporovector was created by ligation of three cut PCR-products. It is designed to allow N-terminal fusion of genes in Freiburg standard ([http://dspace.mit.edu/bitstream/handle/1721.1/45140/BBF_RFC%2025.pdf?sequence=1 RCF25]). Therefore, the gene to be inserted must be cut with XbaI and AgeI and the vector must be digested with XbaI and NgoMIV. By this digest, the RFP cassette will be removed from this vector to allow easy selection for the integration of the new insert. After ligation, there is a scar of six nucleotides between both genes but no restriction site left.
The gene fusion is controlled by the PcotYZ-promoter which was the strongest one in our promoter evaluations. The C-terminal gene part is cgeA, one of the two known spore crust proteins.
The vector is cloned but has not been tested with an insert, yet.
We are working on further Sporovectors to offer also fusion with cotZ as well as C-terminal fusions.
 "
"