Team:TU Darmstadt/Labjournal/Material Science
From 2012.igem.org
(→AFM of FsC) |
|||
| Line 134: | Line 134: | ||
==== AFM of FsC ==== | ==== AFM of FsC ==== | ||
exp1: | exp1: | ||
| - | [File:AFM_FSC.jpg] | + | [[File:AFM_FSC.jpg|220px]] |
| - | [File:Optisch_FSC.png] | + | [[File:Optisch_FSC.png|220px]] |
| - | [File:AFM_Referenz.jpg] | + | [[File:AFM_Referenz.jpg|220px]] |
| - | [File:Optisch_Referenz.png] | + | [[File:Optisch_Referenz.png|220px]] |
exp2: | exp2: | ||
Est13 | Est13 | ||
2 | 2 | ||
| - | [File:3D_EST2_1.png] | + | [[File:3D_EST2_1.png|220px]] |
| - | [File:AFM_EST2_1.jpg] | + | [[File:AFM_EST2_1.jpg|220px]] |
| - | [File:AFM_EST2_1_Amplitude_auto.jpg] | + | [[File:AFM_EST2_1_Amplitude_auto.jpg|220px]] |
| - | [File:AFM_EST2_1_Phase.jpg] | + | [[File:AFM_EST2_1_Phase.jpg|220px]] |
20 | 20 | ||
| - | [File:3D_EST20_2.png] | + | [[File:3D_EST20_2.png|220px]] |
| - | [File:AFM_EST20_2_Amplitude.jpg] | + | [[File:AFM_EST20_2_Amplitude.jpg|220px]] |
| - | [File:AFM_EST20_2_Amplitude_auto.jpg] | + | [[File:AFM_EST20_2_Amplitude_auto.jpg|220px]] |
| - | [File:AFM_EST20_2_Phase.jpg] | + | [[File:AFM_EST20_2_Phase.jpg|220px]] |
50 | 50 | ||
| - | [File:3D_EST50.png] | + | [[File:3D_EST50.png|220px]] |
| - | [File:AFM_EST50.jpg] | + | [[File:AFM_EST50.jpg|220px]] |
| - | [File:AFM_EST50_Amplitude_auto.jpg] | + | [[File:AFM_EST50_Amplitude_auto.jpg|220px]] |
| - | [File:AFM_EST50_Phase.jpg] | + | [[File:AFM_EST50_Phase.jpg|220px]] |
FsC | FsC | ||
| - | [File:3D_FSC.png] | + | [[File:3D_FSC.png|220px]] |
| - | [File:AFM_FSC_1.jpg] | + | [[File:AFM_FSC_1.jpg|220px]] |
| - | [File:AFM_FSC_1_Amplitude_auto.jpg] | + | [[File:AFM_FSC_1_Amplitude_auto.jpg|220px]] |
| - | [File:AFM_FSC_1_Phase.jpg] | + | [[File:AFM_FSC_1_Phase.jpg|220px]] |
Reference | Reference | ||
| - | [File:3D_Referenz_2.png] | + | [[File:3D_Referenz_2.png|220px]] |
| - | [File:AFM_Referenz_1.jpg] | + | [[File:AFM_Referenz_1.jpg|220px]] |
| - | [File:AFM_Referenz_1_Amplitude_auto.jpg] | + | [[File:AFM_Referenz_1_Amplitude_auto.jpg|220px]] |
| - | [File:AFM_Referenz_1_Phase.jpg] | + | [[File:AFM_Referenz_1_Phase.jpg|220px]] |
| + | |||
==== AFM of Est13 ==== | ==== AFM of Est13 ==== | ||
Revision as of 17:16, 24 September 2012
Contents |
Material Science
The main focus of the material science group is the synthesis of polyethylene terephthalate (PET) and structural analoga for the study of the degradation mechanism including atomic force microscopy.
- Synthesis of paranitrophenylesters in presence of triethylamine
- Synthesis of polyethylene terephthalate in presence of sulfuric acid
- Atomic force microscopy
Synthesis of paranitrophnylesters with acyl chlorides in presence of triethylamine
Introducion
The goal of the synthesis is to create paranitrophenylesters with similar polarity and sterical effects as PET-bricks. These are meant to be split enzymatically to deduce an enzymkinetic detecting cutingproducts.
Theory
The synthesis of the esters was achieved by combining acyl chlorides with paranitrophenole in presence of triethylamine. Acetone was used as solvent. The reaction takes 2 h at 0 °C and inert conditions. Triethylamine is used as base to deprotonate paranitrophenol and to catalyze the attack of phenolate at the acyl chloride. It is dissipated by developing hydrogen chloride.
Preparation
Diparanitrophenyl succinate
In a 250 mL triple-neck round-bottom flask with reflux 6.79 g (48.82 mmol/ 2.01 eq.) paranitrophenole and 5.16 g (51.01 mmol/ 2.10 eq.) triethylamine are dissolved in 100 mL acetone under inert conditions and kept at 0 °C using a water/ice mixture. A solution of 1.70 g (10.07 mmol/ 1.00 eq.) succinyl chloride in 50 mL acetone is added dropwise with stirring 2 h at 0 °C. The resulting ester is precipitated in 400 mL distilled water, recrystallized from ethyl acetate three times and dried at 60 °C in a cabinet dryer over night. The yield is 2.3381 g (26.73 % of the theory).
Paranitrophenyl dihydrocinnamate
In a 100 mL triple-neck round-bottom flask with reflux 1.41 g (10.17 mmol/ 1.01 eq.) paranitrophenole and 1.07 g (10.57 mmol/ 1.05 eq.) triethylamine are dissolved in 50 mL acetone under inert conditions and kept at 0 °C using a water/ice mixture. A solution of 3.76 g (24.29 mmol/ 1.00 eq.) dihydrocinnamoyl chloride in 20 mL acetone is added dropwise with stirring 2 h at 0 °C. The resulting ester is precipitated in 200 mL distilled water, filtrated washed with ice cold acetone, stirred in distilled water for 2 h and dried at 60 °C in a cabinet dryer over night. The yield is 1.0623 g (34.69 % of the theory).
Analysis (1NMR-spectroscopy)
Diparanitrophenyl succinate
(300 MHz, CDCl3): δ = 2.99 (2 H), 7.19-7.25 (2 H), 8.18-8.24 (2 H)
Paranitrophenyl dihydrocinnamate
(300 MHz, CDCl3): δ = 2.94-2.97 (2 H), 3.07-3.10 (2 H), 7.18-7.21 (2 H), 7.26-7.35 (5 H), 8.24-8.28 (2 H)
Sources
[http://patentscope.wipo.int/search/en/detail.jsf?docId=WO2009073541&recNum=1&maxRec=&office=&prevFilter=&sortOption=&queryString=&tab=PCT+Biblio: Thermoresponsive arginine-based hydrogels as biologic carriers 11.06.2009]
Synthesis of polyethylene terephthalate in presence of sulfuric acid
Theory
To gain information about different methods of synthesis for Polyethylene terephthalate (PET) and the effect on physical and surface propieties, three experimets are done. The resulting oligo- and polymer is melted, cooled in liquid nitrogen and investigated with AFM. As the synthesis of polyethylene theephthalate is a reversible polycondensation of ethylene glycol and diemthyl terephthalate the developing methanol has to be removed from the reaction by destillation. Concentrated sulfuric acid is used as a catalyst.
Preparation
Conversion to polyethylene terephthalate 1
For the preparation of polyethylene terephthalate 2.73 g (14.05 mmol) of dimethylterephthalate were added to 43.68 mL (781.13 mmol) of ethylene glycol. After the addition of 2-3 drops of concentrated sulfuric acid the solution was heated under reflux at 65 °C for 20 min. After destilation of the Methanol, the resulting yellow solution was cooled for 48 hours at 5°C and filtered. White crystals were precipitated, they were washed with cold hexane and then with a little cold water. The dried crystals are melted at 80°C and poured on a flat metal plate. Which was immediately cooled to -210 °C. The resulting solid was investigated by atomic force microscopy.
Conversion to polyethylene terephthalate 2
For the preparation of polyethylene terephthalate 2.643 g (13.59 mmol) of dimethylterephthalate were added to 0.76 mL (13.59 mmol) of ethylene glycol in 50 mL DMSO. After the addition of 2-3 drops of concentrated sulfuric acid the solution was heated under reflux at 65 °C for 20 min. After destilation of the Methanol, the resulting yellow solution was cooled for 48 hours at 5°C and filtered. White crystals were precipitated, they were washed with cold hexane and then with a little cold water. The dried crystals are melted at 120 °C and poured on a flat metal plate. Which was immediately cooled to -210 °C. The resulting solid was investigated by atomic force microscopy.
Conversion to polyethylene terephthalate 3
For the preparation of polyethylene terephthalate 2.71 g (13.95 mmol) of dimethylterephthalate were added to 0.39 mL (0.69 mmol) of ethylene glycol in 50 mL DMSO. After the addition of 2-3 drops of concentrated sulfuric acid the solution was heated under reflux at 65 °C for 20 min. 0.39 mL (0.69 mmol) of ethylene glycol were added dropwise over 30 min. After destilation of the Methanol, the resulting yellow solution was cooled for 48 hours at 5 °C and filtered. White crystals were precipitated, they were washed with cold hexane and then with a little cold water. The dried crystals were melted at 170 °C. While reaching the temperature of 170 °C the crystals suplimated imediatly leading to a total loss of product.
Atomic Force Microscopy (AFM)
Theory
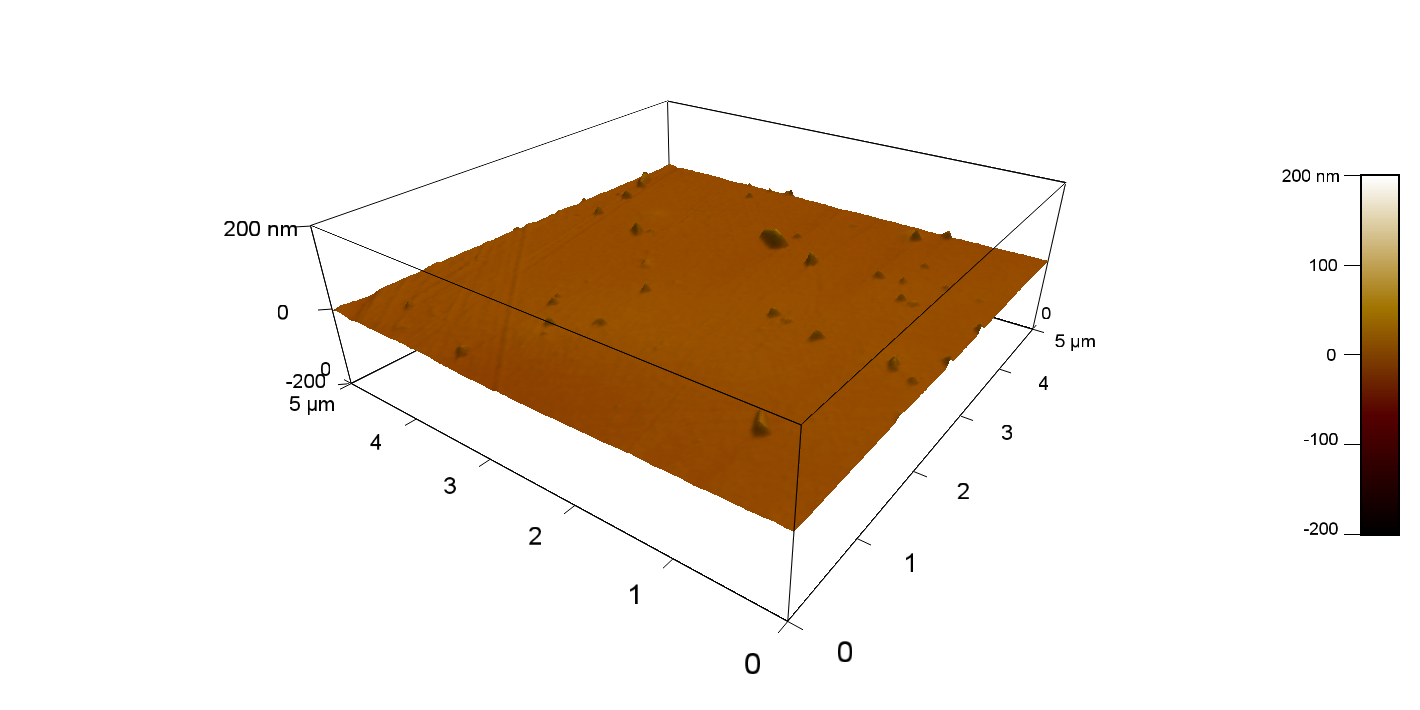
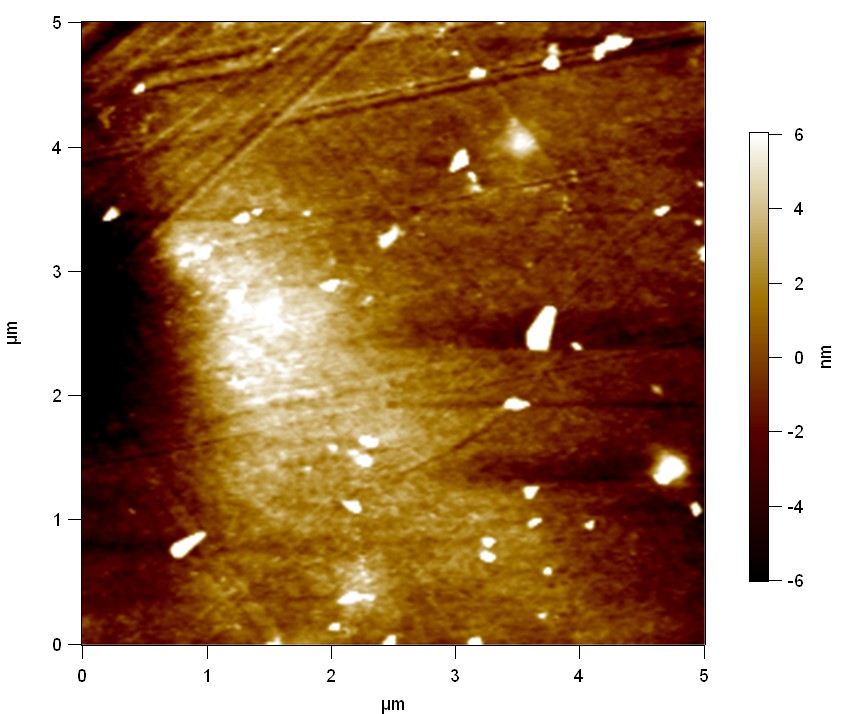
AFM of FsC
exp1:
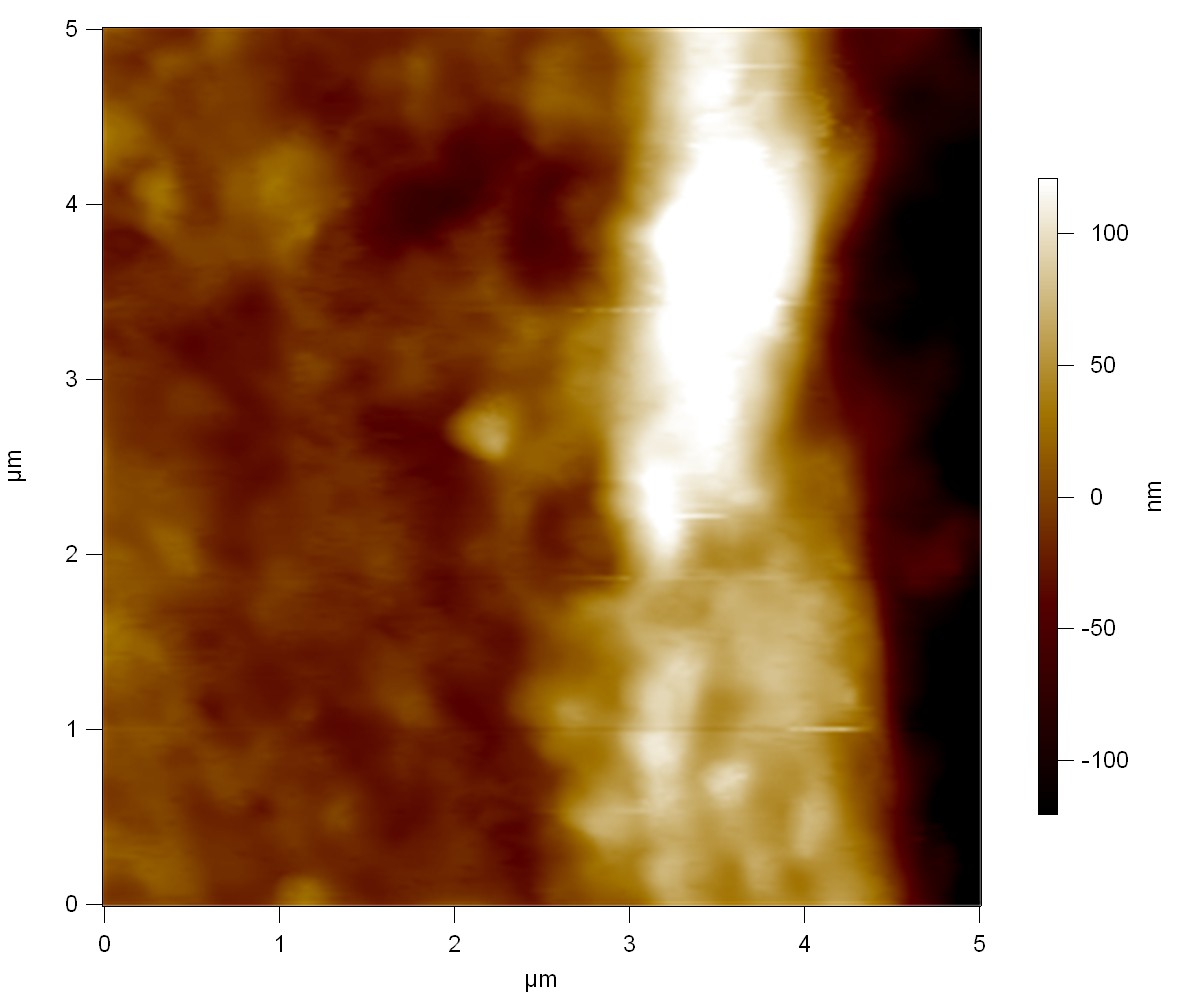
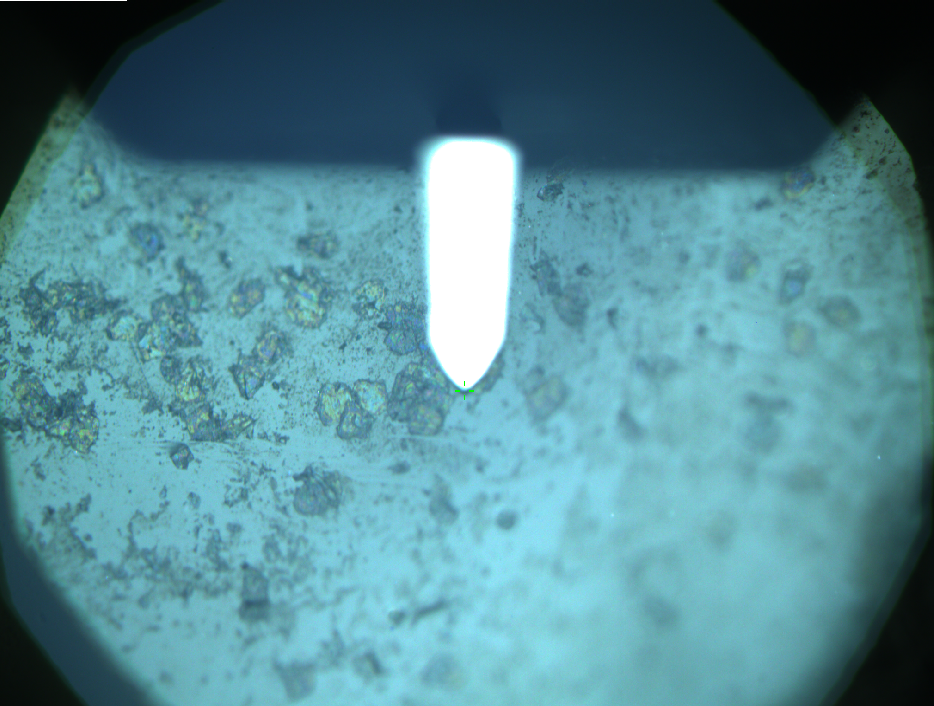
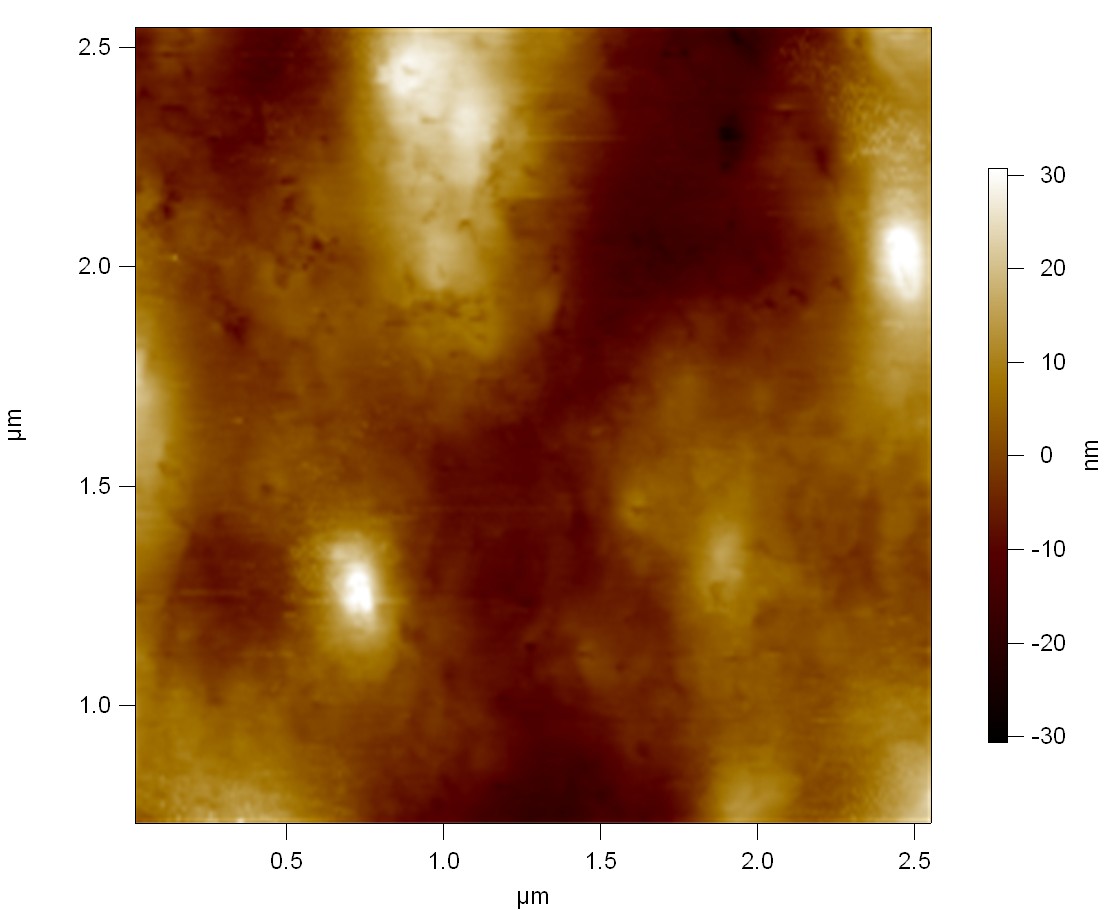
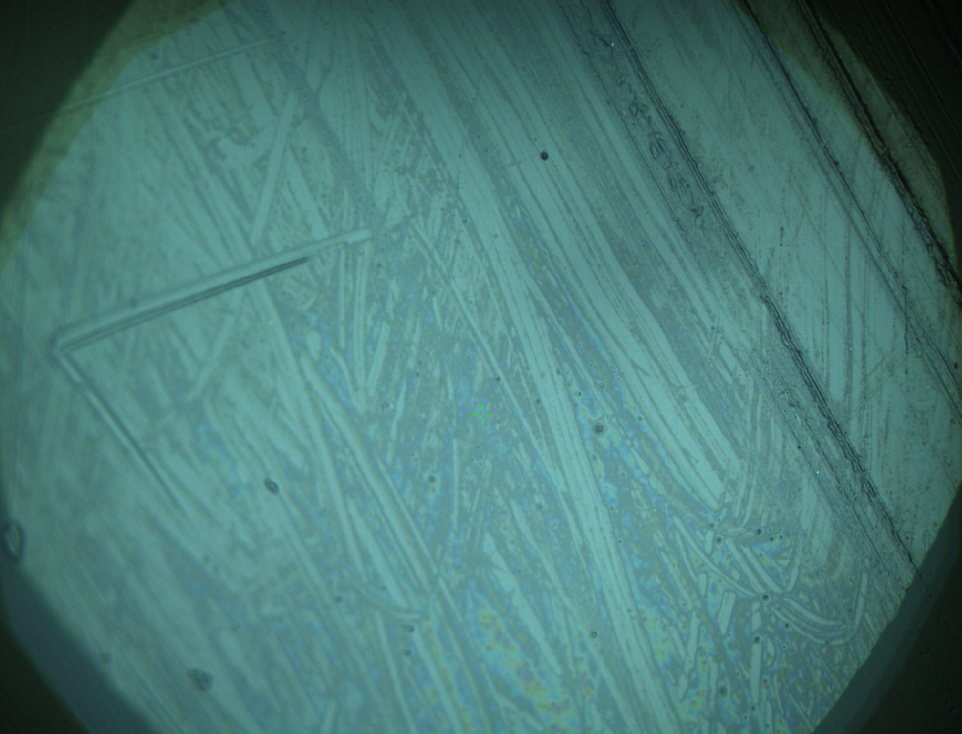 exp2:
Est13
2
exp2:
Est13
2
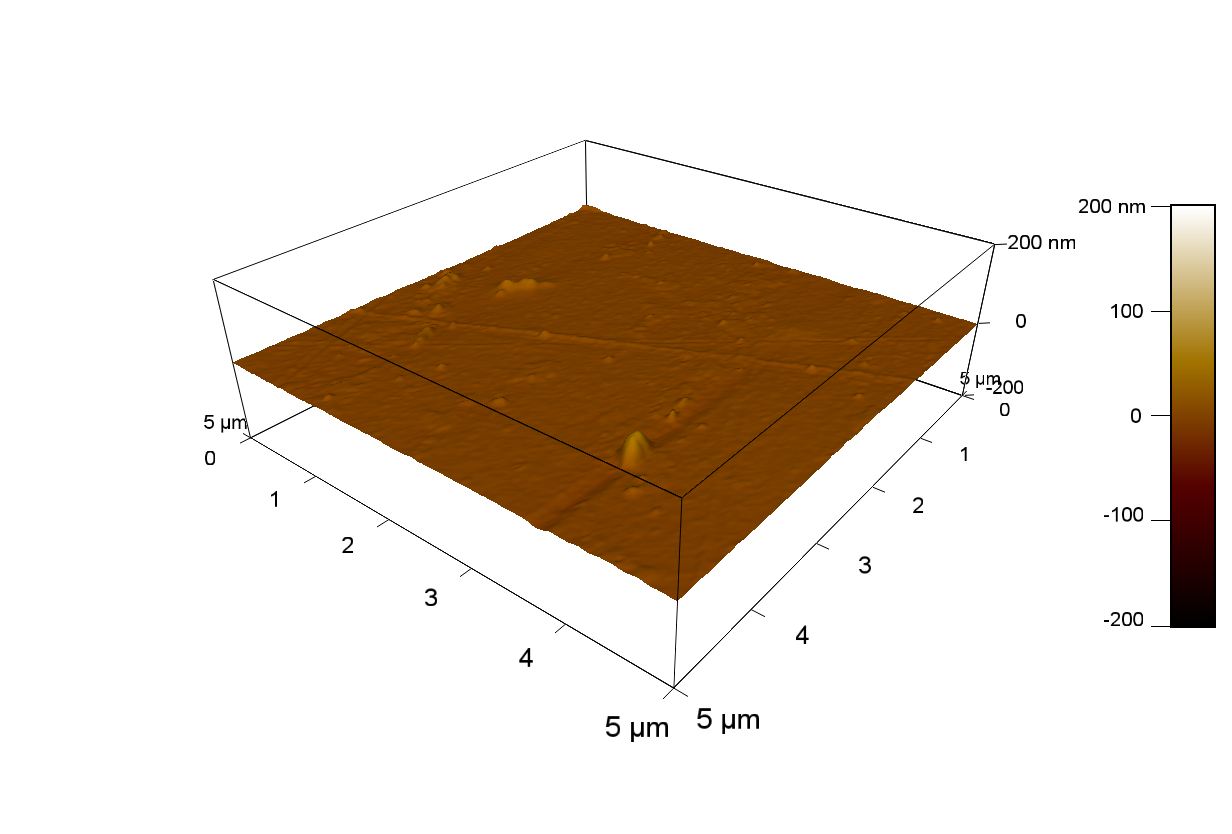
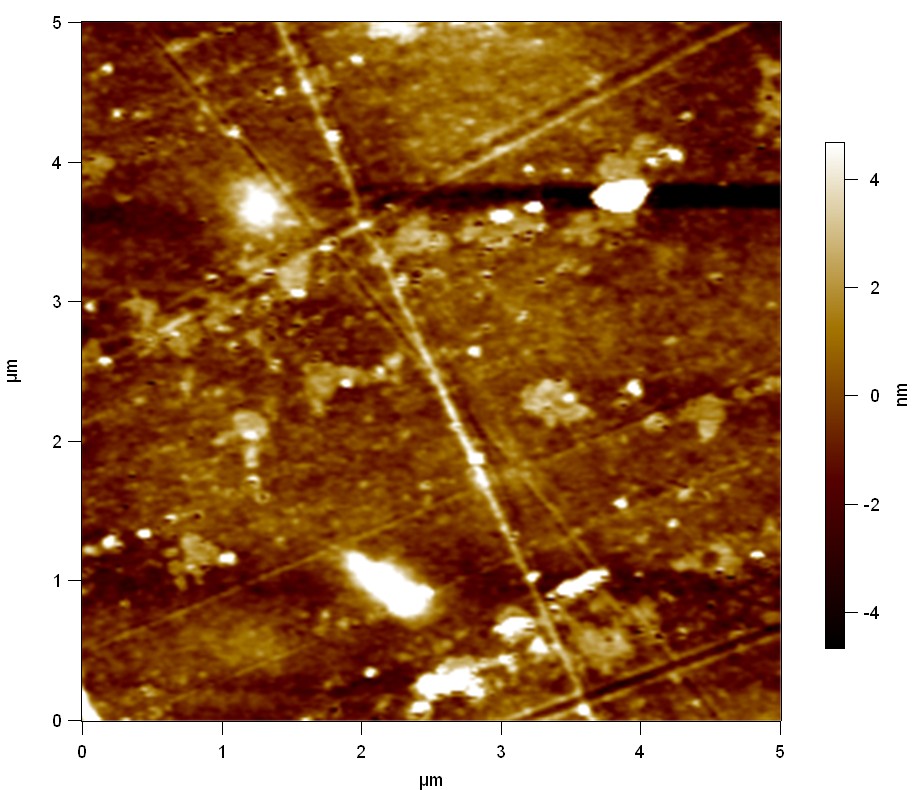
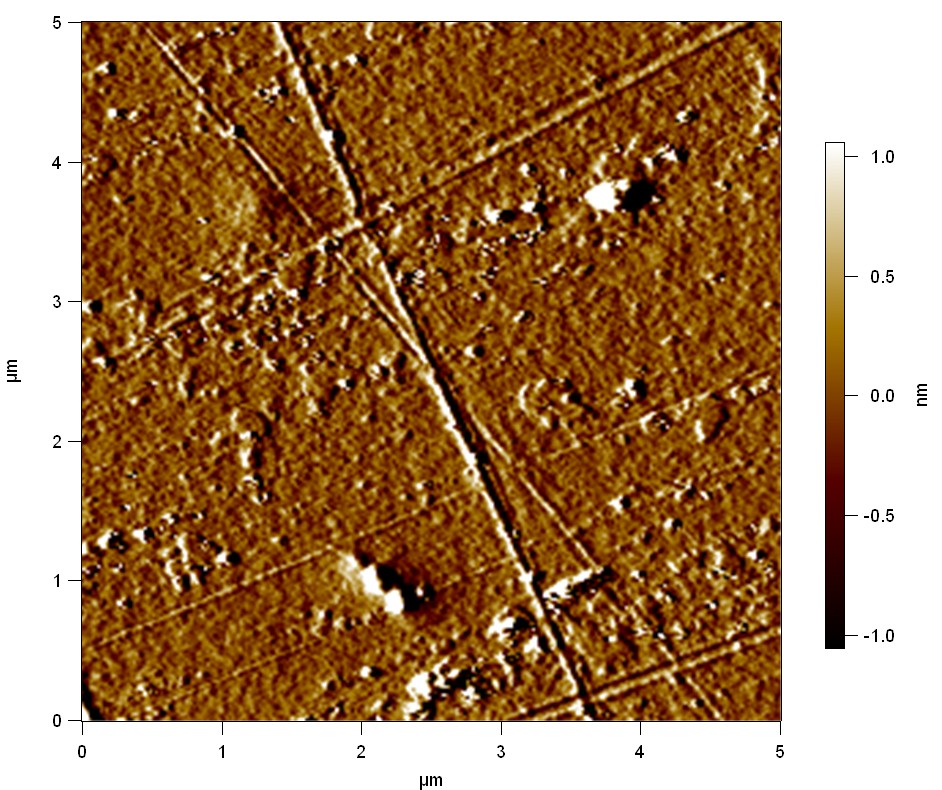
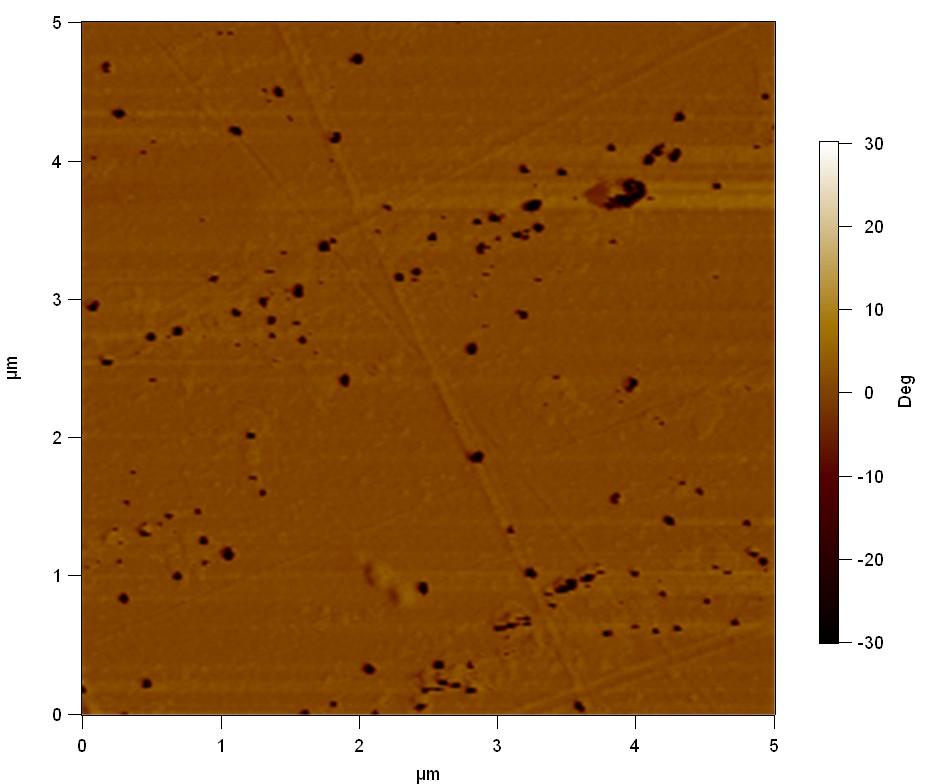 20
20
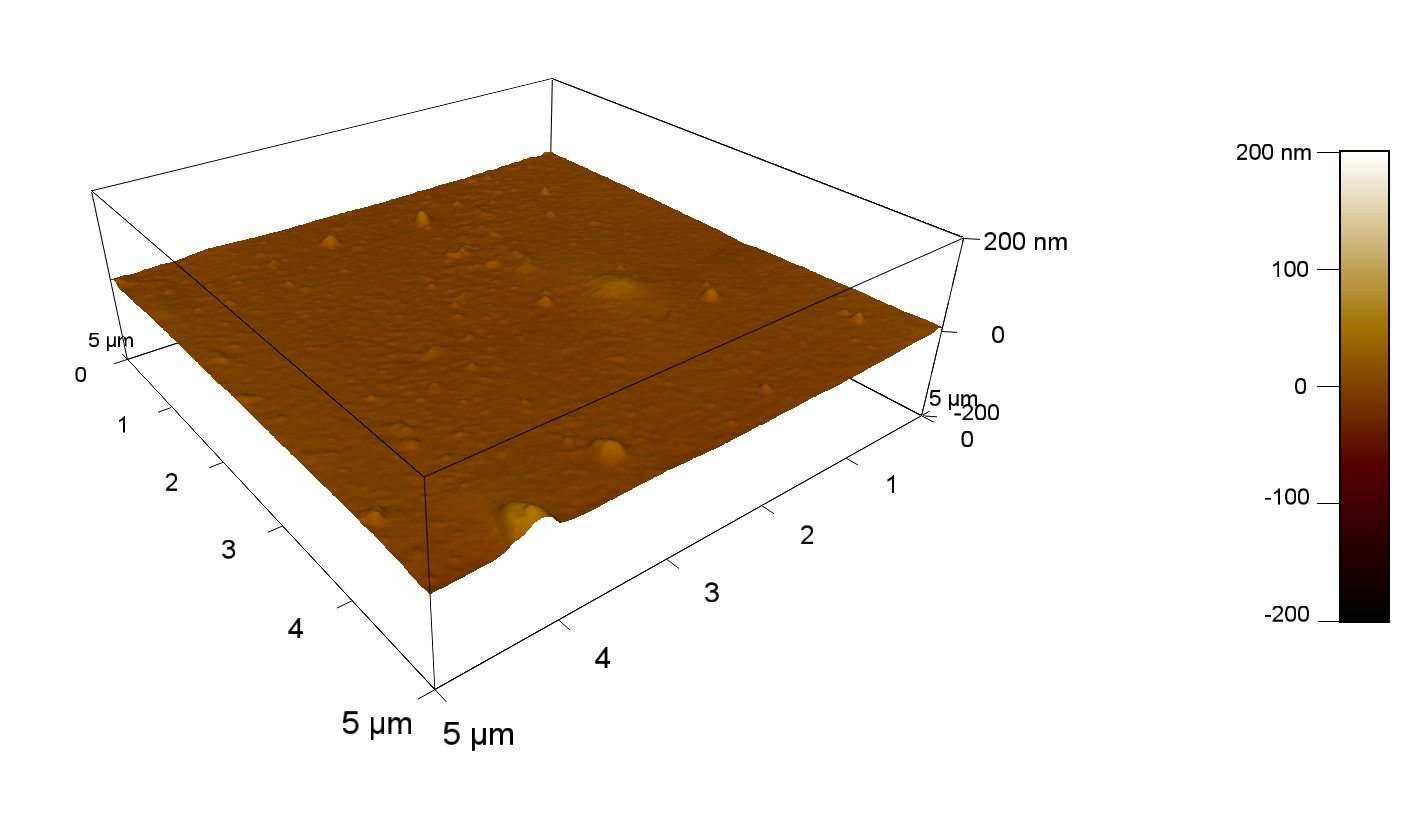
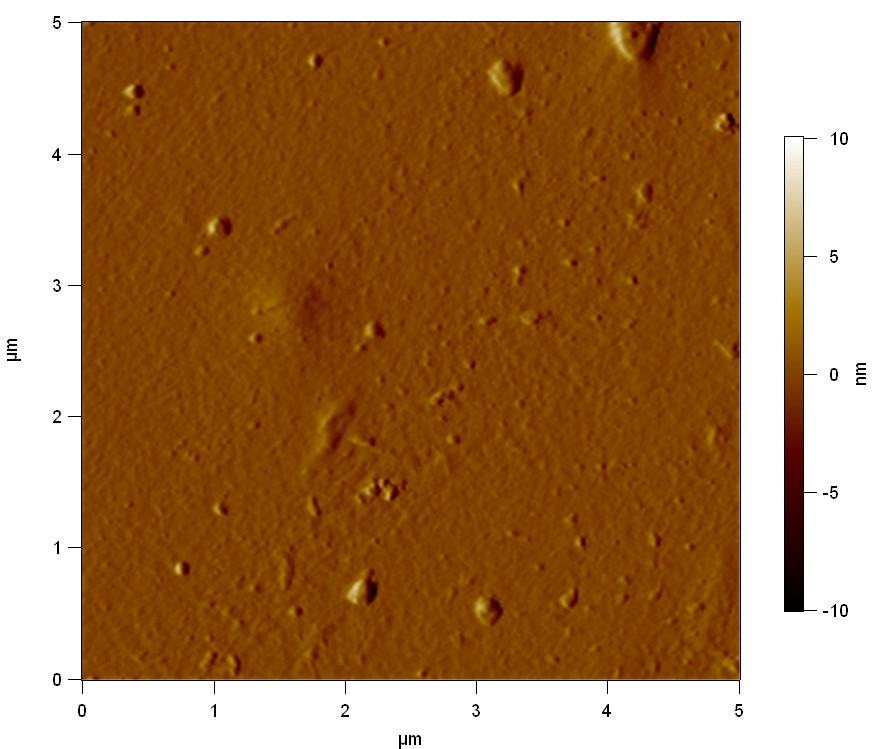
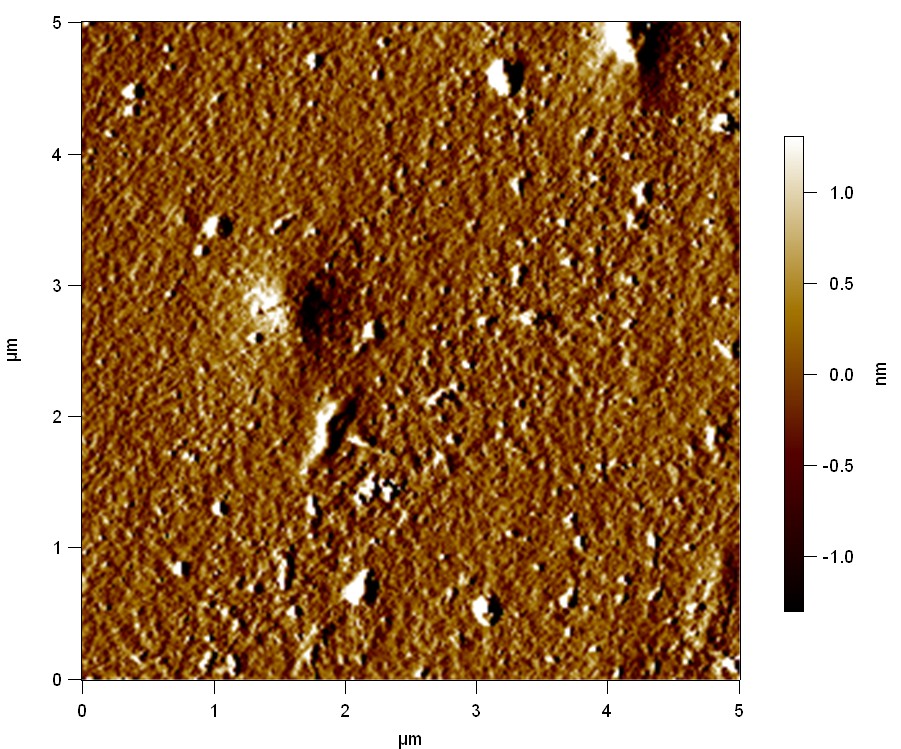
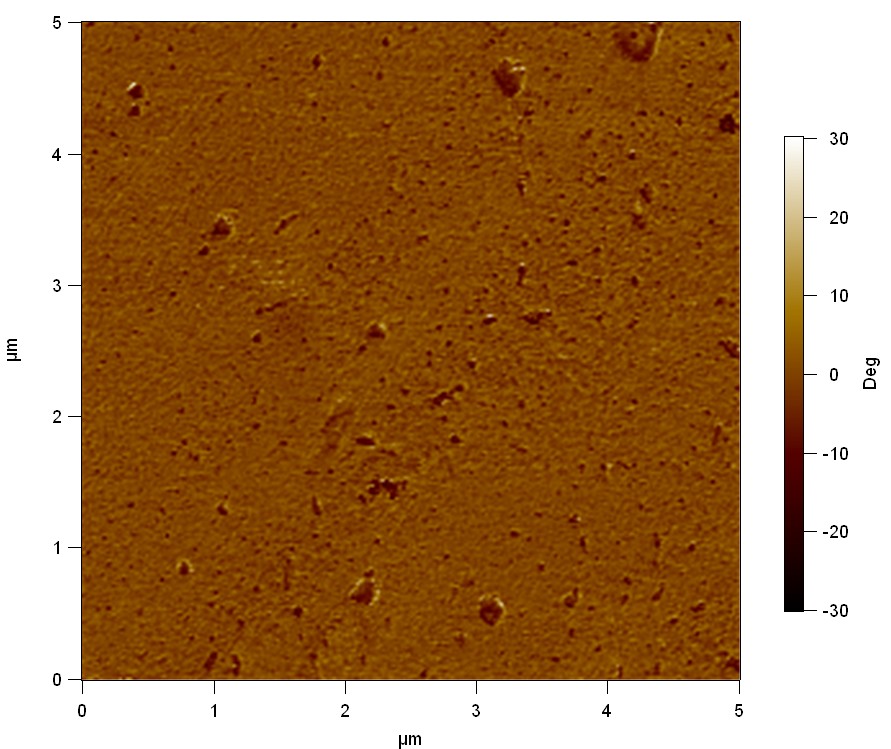 50
50
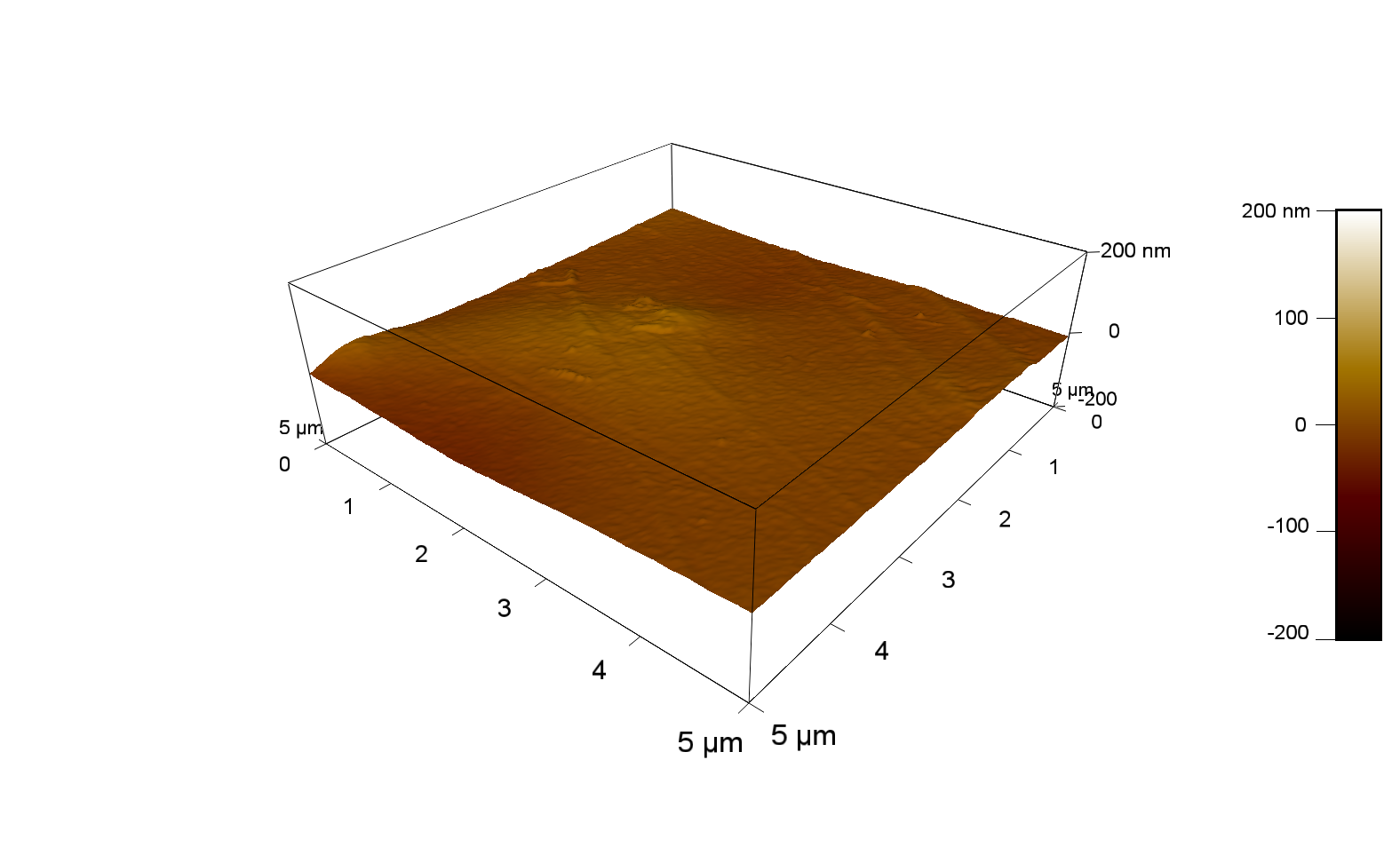
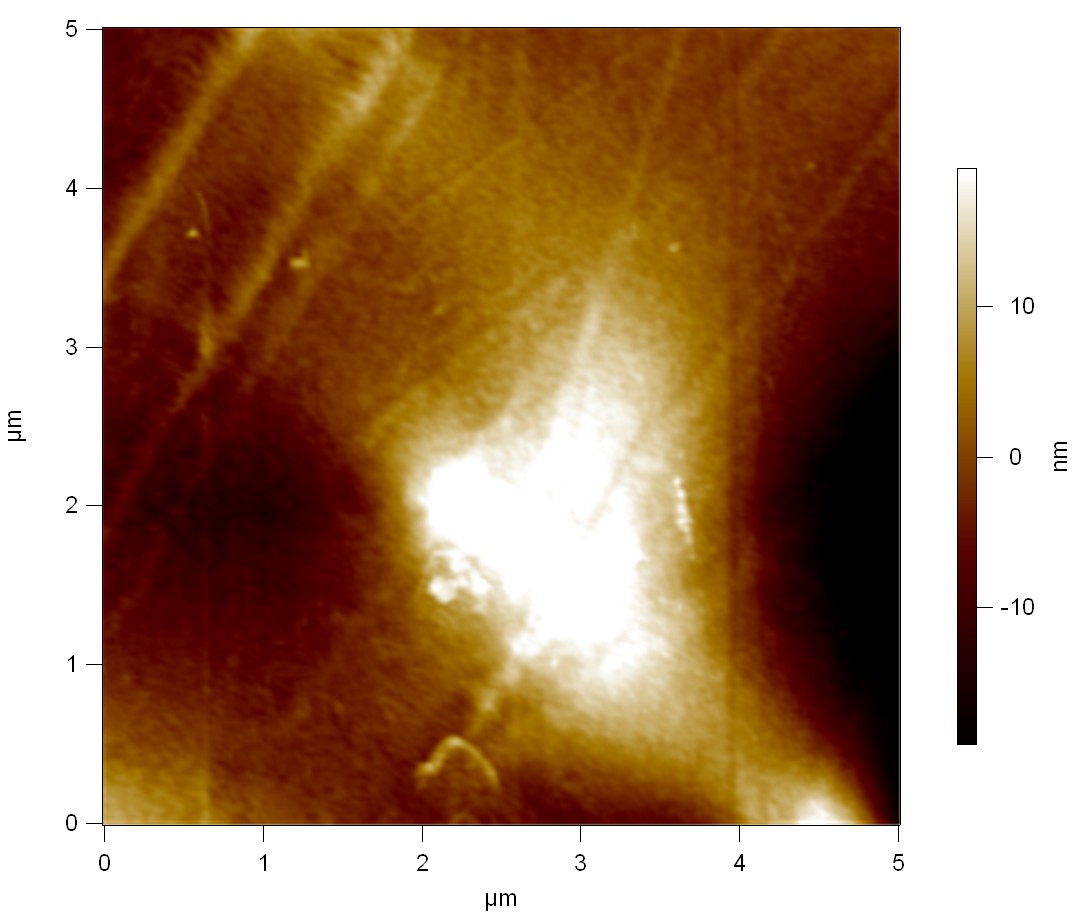
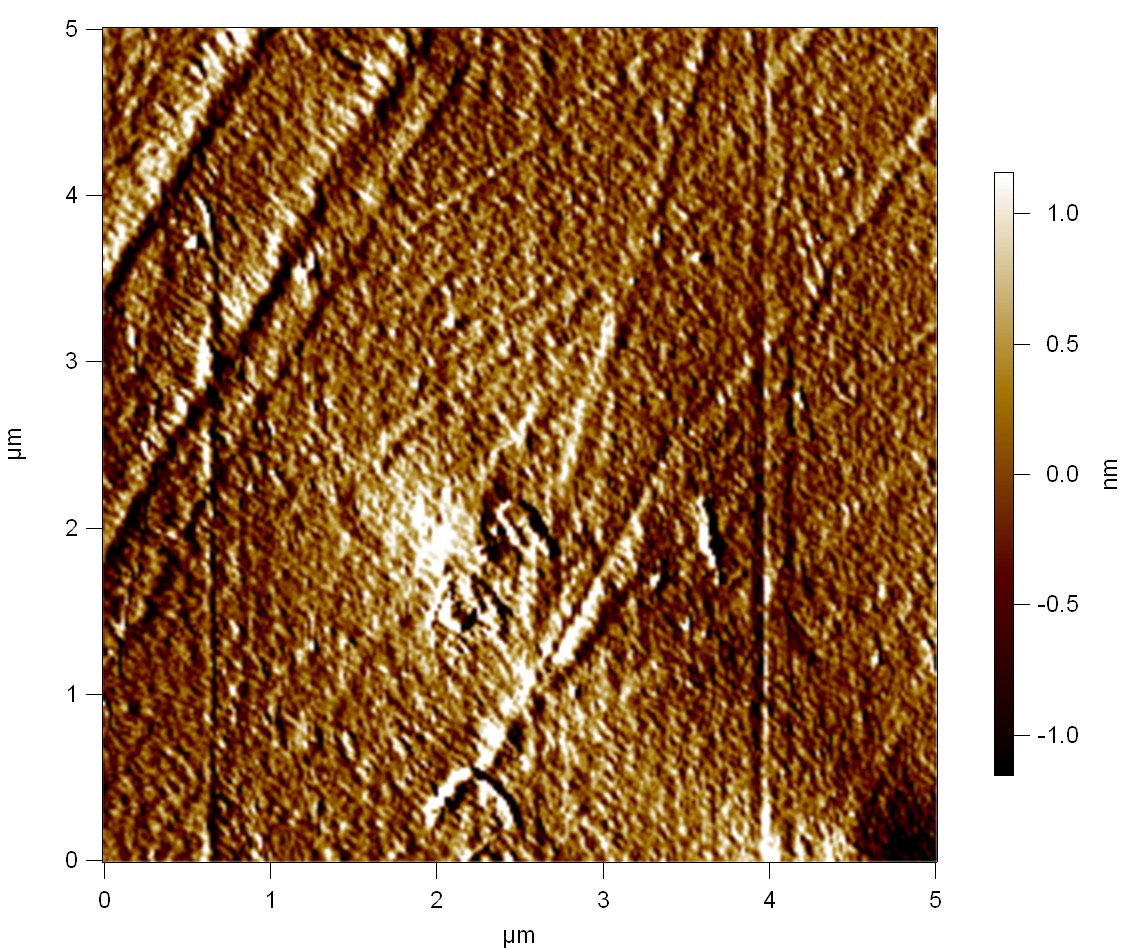
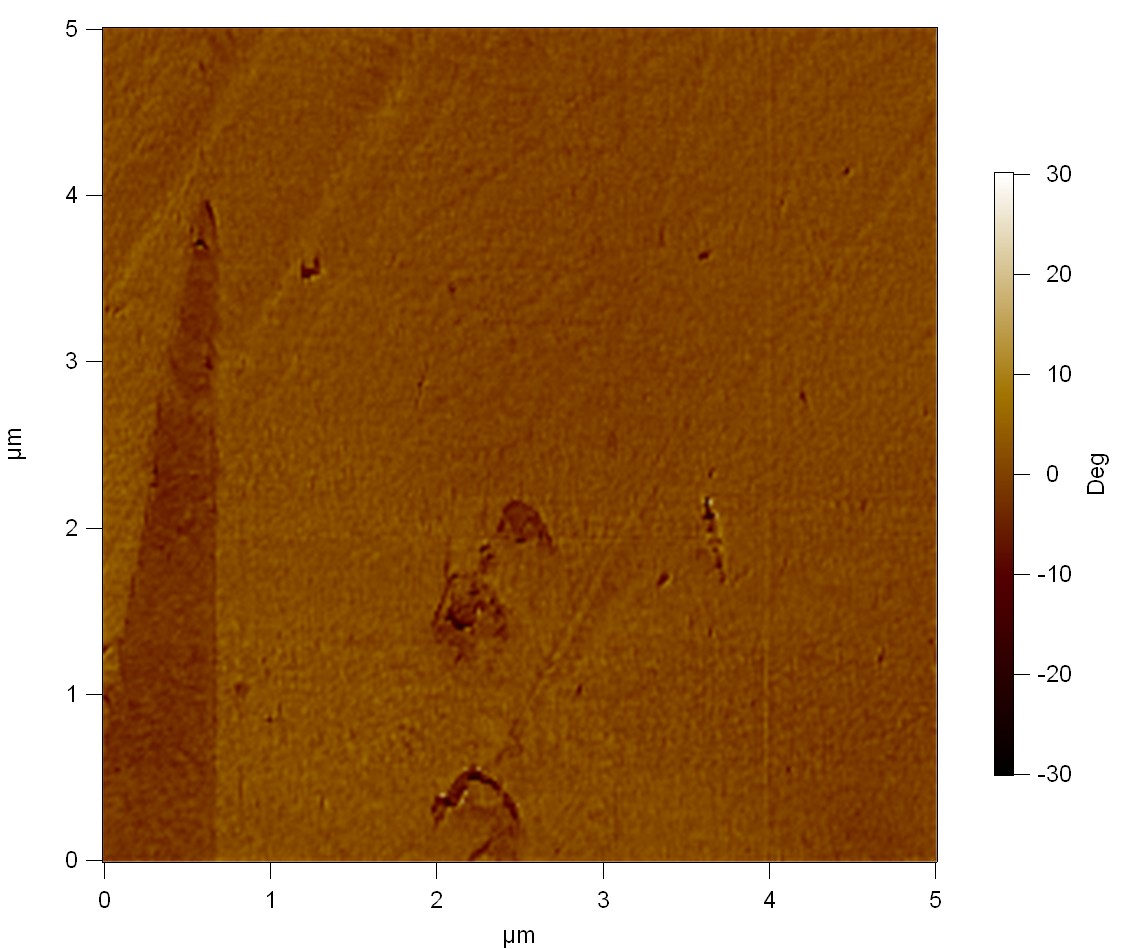 FsC
FsC
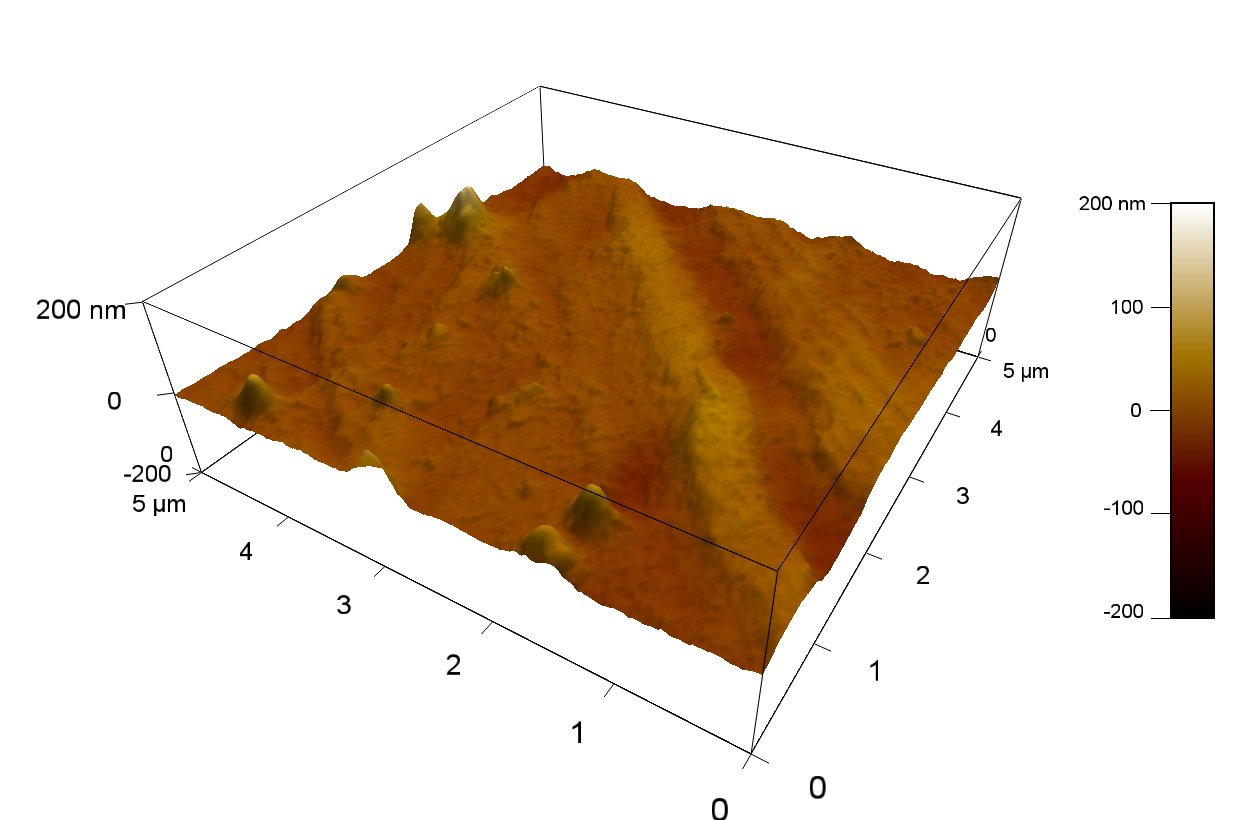
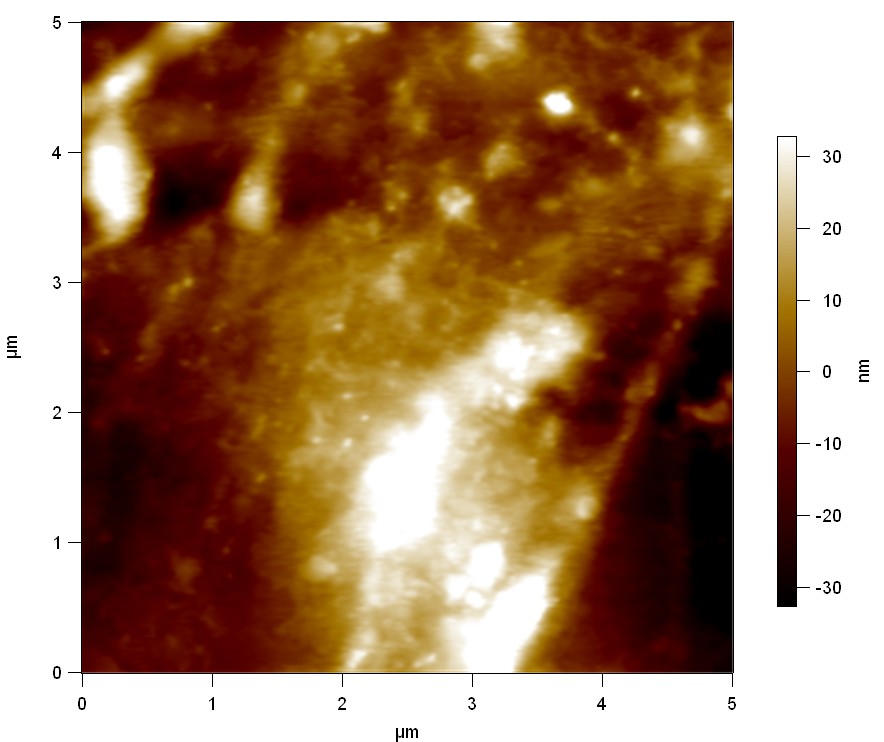
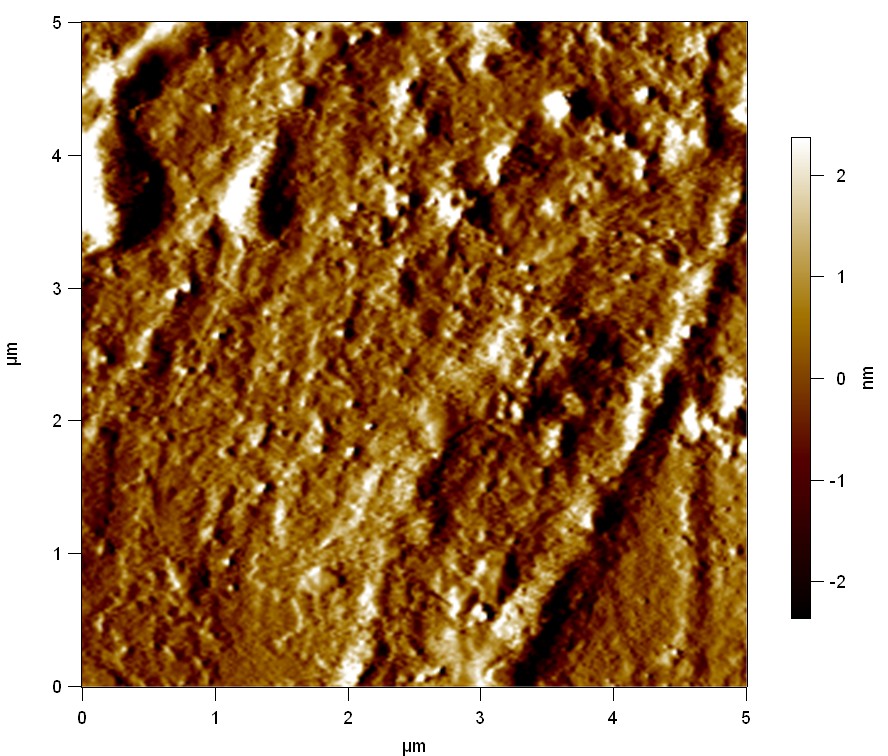
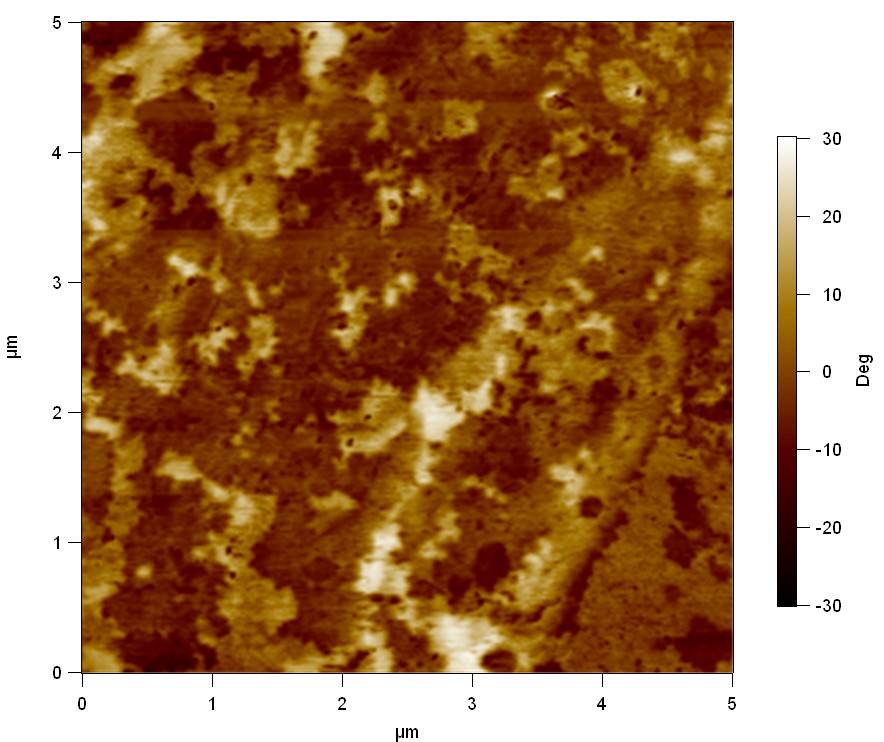 Reference
Reference
 "
"