Team:UNITN-Trento/Project/CrustAway
From 2012.igem.org
| Line 1: | Line 1: | ||
| + | |||
<html> | <html> | ||
<head> | <head> | ||
| Line 172: | Line 173: | ||
<h3>How do we test the expression of our enzymes?</h3> | <h3>How do we test the expression of our enzymes?</h3> | ||
<p>We used the Gibson assembly method to create two sfGFP-tagged devices that were useful reporter to verify the expression of our genes.</p> | <p>We used the Gibson assembly method to create two sfGFP-tagged devices that were useful reporter to verify the expression of our genes.</p> | ||
| - | <div style="width: 700px; margin: 0 auto;"><img style="width: | + | <div style="width: 700px; margin: 0 auto;"><img style="width: 700px;" src="http://www.science.unitn.it/~igem/img/project/ConstructsGFP.jpg" /></div> |
<div style="text-align: center;" id="results"><a href="javascript:void(0);"><h3>GO TO OUR RESULTS</h3></a></div> | <div style="text-align: center;" id="results"><a href="javascript:void(0);"><h3>GO TO OUR RESULTS</h3></a></div> | ||
</div> | </div> | ||
Revision as of 08:04, 18 September 2012
Crust Away
INTRODUCTION
Have you ever noticed that ugly black crust found on precious monuments and statues? Here in Italy, we surely have it, and we’d like to find a way to return our monuments to their natural, beautiful state.

Our idea is to create a bioremediation kit to help every sculptor, restorer, marble cutter or anyone interested in removing that ugly and harmful black crust layer from their precious calcareous stones. A lot of chemical and mechanical approaches are already off the shelf, and someone also exploited in the past Sulfur Reducing Bacteria (SRB) to eliminate the trapping gypsum matrix (Capitelli et al. 2007).
However all of these methods show some weak points:
- Chemical and mechanical methods are too invasive and they risk to damage the underlying and precious marble surface; We have met with experts in the restoration field and learned about the criteria to define a good restoration.
- Chemical methods have also health related risks. Workers, artists, restorers too often use chemicals to clean marble stones in unsafe conditions. We have met with one of the many local artists and reported our impressions on his methods to clean marble in the Art & Science section of our Wiki.
- Natural SRB, which have been used in a few cases, instead need anaerobic conditions and they constitutively express the enzymes required for sulphur reduction, thus not leaving the choice to the operator to control the rate and amount of sulphur reduced.
- Work in AEROBIC conditions: this is a breakthrough in the field of SRB!
- Should be CONTROLLED and MODULATED as needed.
- Should NOT be INVASIVE and work selectively against the gypsum matrix without touching the calcareous surface.
- Should be CHEAP and EASY TO APPLY. There will be no more need of expensive chemical and trained workers that have to work many hours to clean our statues and monuments.
To summarize, with our biological system we want to mix the strengths of all the available methods and eliminate their weak points to propose an infallible and very safe method to clean precious marble pieces!
How does the black crust form?
One of the main factors in the formation of the black crust is the atmospheric pollution. The burning process of fossil fuels leads to an increase in the concentration of some acid gases in the atmosphere. In particular SO2 when reacts with water induces the transformation of calcite (CaCO3, present in the stone substrate) into gypsum (CaSO4 ·2H2O), which precipitates with inclusions of carbon particulate matter. Smog particles are also able to absorb gas pollutants on their surfaces, resulting in “dry deposition” of pollutant.
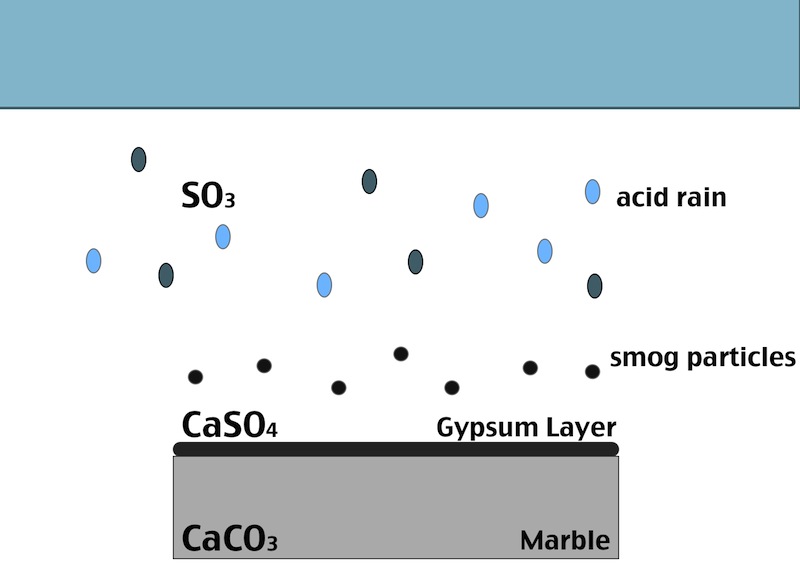
Black crust are usually found on areas of the stone sheltered from rainfall, although still in presence of capillary water flowing through the pores. Calcium ions migrate to the surface of the stone during gypsum formation, leading to formation of cavities beneath the stone that weakens the stone’s integrity. The degradation process indeed affects both the stone conservation and appearance:
- When the formed gypsum is washed away it takes some of the stone particles with it, causing at first loss of detail, but eventually leading, again, to a loss of structural integrity.
- In areas sheltered from rainwater, though, gypsum crusts remain. When combined with particulate matter from the atmosphere, gypsum creates the so called black crust.
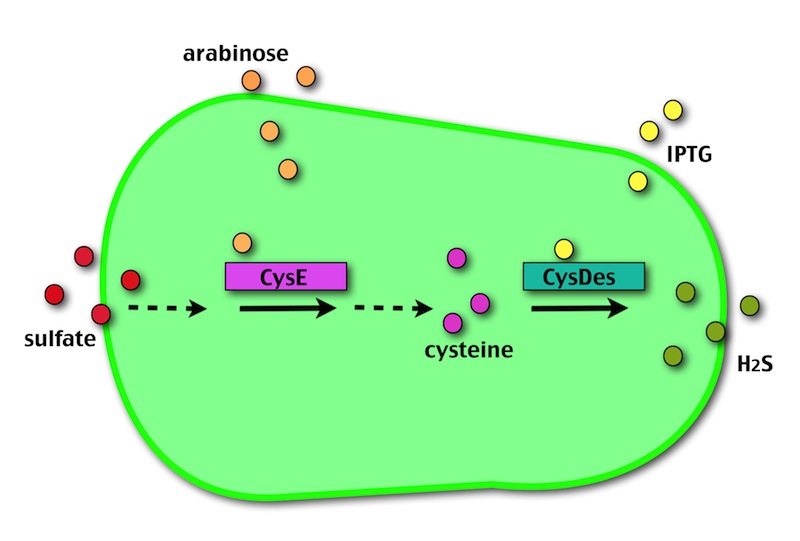
OUR METHOD
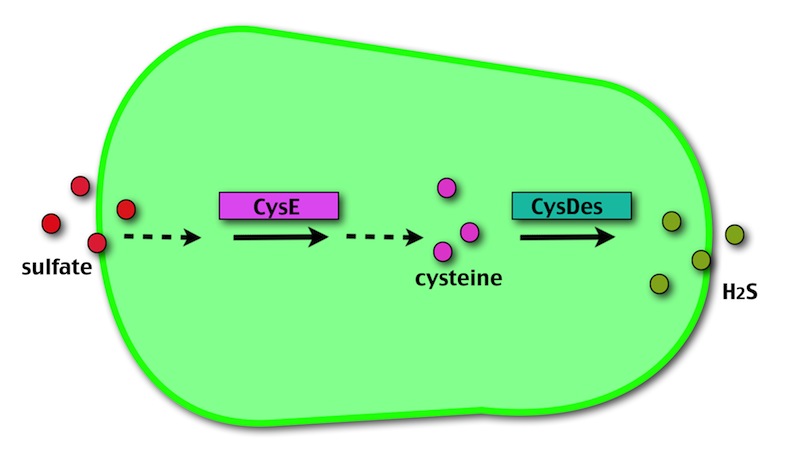
Our method exploits an engineered E. coli to reduce aerobically the sulphuric gypsum layer in a controlled and modulated manner. In 2001 the Keasling group has engineered bacteria to reduce sulphate aerobically and precipitate metals from water taking advantage of the production of sulfidric acid as one of the bioproducts of sulphur reduction. We took inspiration from this work and developed two new BioBrick devices directed to overproduce cysteine and then convert it to sulphide with the ultimate goal of dissolving the sulphate layer deposited on the blackened stones.
Who are the players?

- CysE is a Serine acetyltransferase that mediates conversion of L-serine to a precursor of L-cysteine in E.coli. More precisely we have used a mutant CysE (M256I) that has enhanced activity in that its enzymatic activity is less sensitive to feedback inhibition by cysteine.
- CysDes is a Cysteine Desulfhydrase, first isolated in Treponema denticola, an anaerobic organism involved in periodontal diseases. It is an aminotransferase that converts cysteine into pyruvate, ammonia, and hydrogen sulphide.
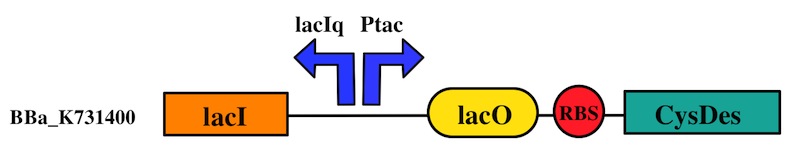
How do we control the expression of our devices?
The expression of CysE is controlled by an arabinose inducible cassette (our part BBa_K731201). Our composite device (BBa_K731030) is composed by araC –pBad, and strong RBS followed by M256I CysE.
The pBAD promoter is activated in the presence of L-arabinose. L-arabinose binds to the AraC protein and inactivates its inhibitory function, allowing the RNA polymerase to recognize the pBAD promoter and start the transcription of CysE.
CysDes was placed downstream of an IPTG inducible cassette (our part BBa_K731300). The expression of CysDes is therefore controlled by a system composed of: LacI (repressor protein) and a lacIq promoter followed by a strong hybrid promoter (Ptac), a Lac Operator and a strong RBS. Addition of IPTG inactivates the LacI repressor, thus allowing the expression of our gene of interest CysDes.
The possibility of controlling simultaneously the expression of our two devices (i.e. by adding arabinose and/or IPTG) makes our system more SAFE, CONTROLLABLE and MODULAR.
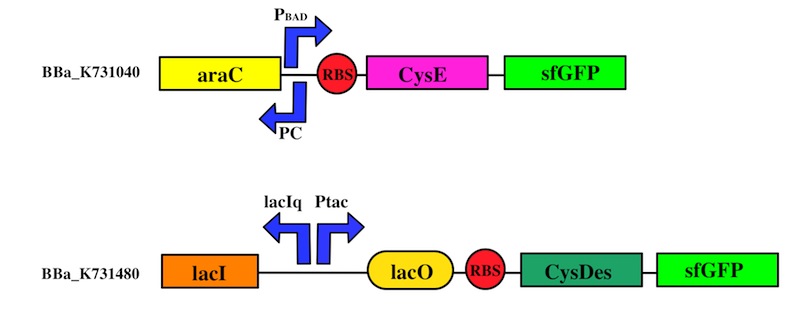
How do we test the expression of our enzymes?
We used the Gibson assembly method to create two sfGFP-tagged devices that were useful reporter to verify the expression of our genes.
 "
"