Team:Wageningen UR/ObtainingthePoleroVLP
From 2012.igem.org
(→Isolation and BioBricking of the potato leafroll virus (PLRV) coat protein.) |
m (→Isolation and BioBricking of the potato leafroll virus (PLRV) coat protein.) |
||
| Line 9: | Line 9: | ||
The PLRV coat protein can thereafter be ‘’BioBricked’’. This brick can be expressed in Escherichia coli and produced monomers can be isolated and purified. | The PLRV coat protein can thereafter be ‘’BioBricked’’. This brick can be expressed in Escherichia coli and produced monomers can be isolated and purified. | ||
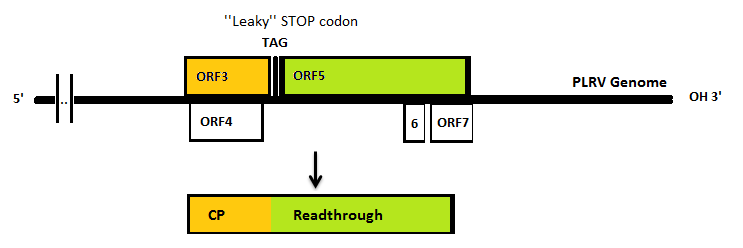
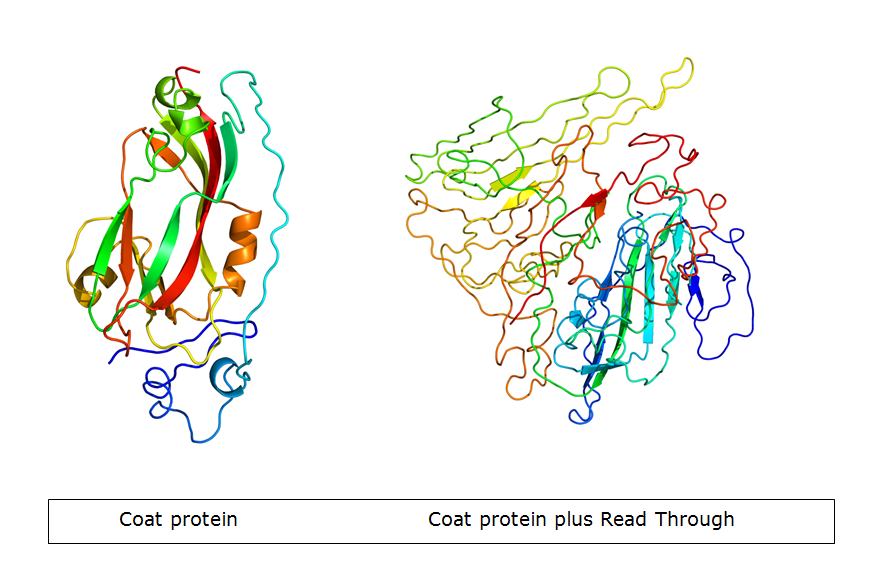
| - | In nature, the stopcodon of the coat protein is sometimes ‘’missed’’, which results in a 70kDa readthrough product. This is considerably larger than the 23kDa coat protein on its own. When the coat protein as well as the coat protein plus readthrough are assembled together to form the virus capsid, spikes are formed on the capsid. These | + | In nature, the stopcodon of the coat protein is sometimes ‘’missed’’, which results in a 70kDa readthrough product. This is considerably larger than the 23kDa coat protein on its own. When the coat protein as well as the coat protein plus readthrough are assembled together to form the virus capsid, spikes are formed on the capsid. These spikes, according to literature, have a function in the transmission by aphids, primarily the green peach aphid, ''Myzus persicae''. We expect that these spike can be changed without having a large effect on VLP formation. This, thus, seems a good strategy to build in either E-coils or K-coils, described in the general project introduction. |
Virus like particles (VLPs) are being formed either with or without readtrough proteins. The expression of coat protein monomers, needed for VLP formation, has never been done in E.coli. If this step is successful, further modifications on the VLP’s will be done. | Virus like particles (VLPs) are being formed either with or without readtrough proteins. The expression of coat protein monomers, needed for VLP formation, has never been done in E.coli. If this step is successful, further modifications on the VLP’s will be done. | ||
Revision as of 11:35, 10 September 2012
Isolation and BioBricking of the potato leafroll virus (PLRV) coat protein.
The Polero (potato leaf roll virus, PLRV) virus coat proteins can be used as building blocks to form VLP’s. PLRV is a positive sense RNA virus (group IV). Viruses from this group have their genome directly utilized as if it were mRNA. Ribosomes from the host cell translate their genome into a protein, with RNA-dependent RNA polymerase as one of it. This means that to isolate the coat protein gene we need to isolate the RNA of infected potato plants which would include the viral RNA.
After obtaining the coat protein gene of PLRV, the PCR product can be sent to be sequenced. The sequence of our isolate together with other isolates will give us information about the conserved regions in the gene. Alignment experiments can be performed, also together with data of other members of the Luteoviridae. The PLRV coat protein can thereafter be ‘’BioBricked’’. This brick can be expressed in Escherichia coli and produced monomers can be isolated and purified.
In nature, the stopcodon of the coat protein is sometimes ‘’missed’’, which results in a 70kDa readthrough product. This is considerably larger than the 23kDa coat protein on its own. When the coat protein as well as the coat protein plus readthrough are assembled together to form the virus capsid, spikes are formed on the capsid. These spikes, according to literature, have a function in the transmission by aphids, primarily the green peach aphid, Myzus persicae. We expect that these spike can be changed without having a large effect on VLP formation. This, thus, seems a good strategy to build in either E-coils or K-coils, described in the general project introduction. Virus like particles (VLPs) are being formed either with or without readtrough proteins. The expression of coat protein monomers, needed for VLP formation, has never been done in E.coli. If this step is successful, further modifications on the VLP’s will be done.
Aim: Our primary aim is to obtain self-assembling VLP’s from PLRV in E. coli. If we succeed, we can attempt to modify the spike on the outside.
Progress:
- RNA isolation of potato leaf tissue
- Reverse transcriptase reaction to obtain cDNA
- PCR on cDNA using coat protein primers
- Addition of Prefix and Suffix to CP genes
- Transformation of the gene, inserted into iGEM pSB1C3 backbone, into E.coli
- Sequencing of coat protein gene
- Submission to Registry
- Luteoviridae coat protein/readtrough analogy experiments
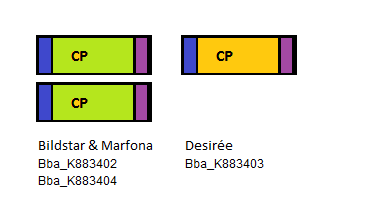
Four PLRV parts were sequenced: three PLRV coat proteins from different potato cultivars, one PLRV coat protein with a Histidine-tag added to the N-terminal. The three bare PLRV coat proteins were confirmed to be positioned nicely within the iGEM prefix and suffix. Unfortunatelly the sequence results showed the absence of the PstI restriction site in the suffix. The three different PLRV coat protein BioBrick parts were submitted to the Registry with the following accession numbers: BBa_K883402, BBa_K883403 and BBa_K883404.
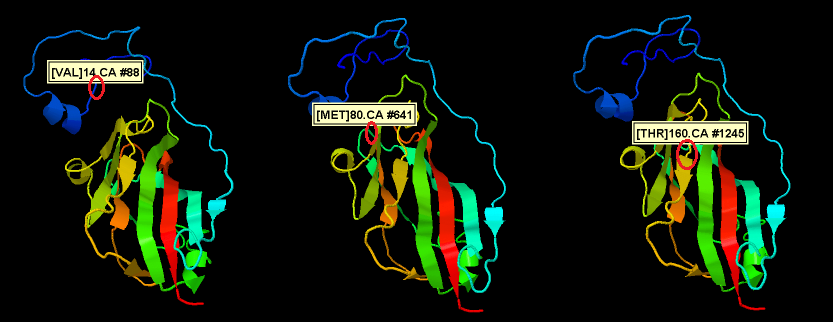
Alignment of the different PLRV isolates shows 9 SNP’s but not all SNP’s result in a different amino acid (only 3). The position of these amino acid changes if shown in the figure below.
Future work
- Isolation of monomers and formation of VLP’s
- VLP analysis/characterisation
We inserted an IPTG inducable promotor upstream the PLRV CP gene. We performed 2 attempts to express the PLRV coat protein in E.coli but these were not succesfull. We obtained PLRV antibodies to use with Western blot to check for PLRV CP expression. Right now, we focus on getting the PLRV CP expressed. After succesfull expression we will try to isolate formed VLP's.
Further progress will be posted asap;)
 "
"