Team:Wageningen UR/Journal/week5
From 2012.igem.org
m |
Hanyue0731 (Talk | contribs) (→Lab work) |
||
| (16 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
== Office work == | == Office work == | ||
| - | + | <p align="justify"> | |
| - | This week a lot of new information | + | This week a lot of new information has been added to the wiki. There is now a team description, the journal has been updated for the most part and a lot of the protocols have been added. We've also worked further with the quaternary structure program, with minor successes. Furthermore the logo is finished and will be uploaded on the wiki next week. Thursday evening we started thinking how to call our project, but the group is still divided. On Friday we worked with the data of the Electron Microscopy and Dynamic Light Scattering and send the results and conclusions to our advisors. Hopefully the results will be posted next week on the wiki. |
| - | + | </p> | |
'''Wiki page''' | '''Wiki page''' | ||
'''Munich''' | '''Munich''' | ||
| - | ''' | + | '''Modeling''' |
| Line 21: | Line 21: | ||
'''CCMV test protocol:''' | '''CCMV test protocol:''' | ||
| + | |||
| + | Monday: | ||
| + | *Western Blotting | ||
| + | *prepared 10x Towbins electrotransfer buffer | ||
| + | *sandwich build-up: | ||
| + | **case (white side) | ||
| + | **sponge | ||
| + | **Whatman paper | ||
| + | **Whatman paper | ||
| + | **nitrocellulose membrane | ||
| + | **SDS gel | ||
| + | **Whatman paper | ||
| + | **Whatman paper | ||
| + | **sponge | ||
| + | **case (black side) | ||
| + | * all layers carefully soaked in 1x electrotransfer buffer | ||
| + | * run at 0.15A overnight in 4°C cold room | ||
| + | * missed a secondary antibody, no result available | ||
| + | |||
Tuesday: | Tuesday: | ||
| - | |||
*Prepare samples for ultracentrifuge according to the protocol | *Prepare samples for ultracentrifuge according to the protocol | ||
*Starting ultra centrifuge, 45000 RPM for 3 hours | *Starting ultra centrifuge, 45000 RPM for 3 hours | ||
| + | <p align="justify"> | ||
| + | This was a very productive week, with good results. After the dialysis of last week, We began to prepare the CCMV samples for ultracentrifuge. After the preparation, the ultracentrifuge was started and the three hour wait began. Afterwards We made an appointment for Thursday for the Electron Microscopy and the Dynamic Light Scattering machine. | ||
| + | </p> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| + | Wednesday: | ||
| + | * Started second CCMV VLP production | ||
| + | * Started PCR for CCMV wt, CCMV delta 25 variances with pre and suffix | ||
| + | * Started PCR for CCMV his tag and CCMV delta 25 his tag variances with pre and suffix | ||
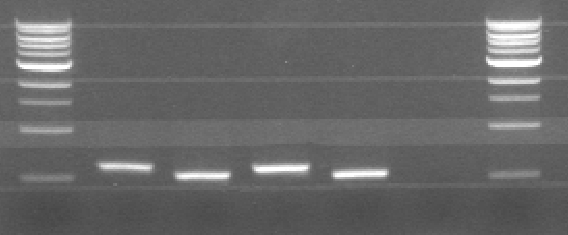
| + | [[File:Pcr31-5.PNG|300px|center|thumb|Figure 1: Agarose gel of PCR. From left to right: CCMV wt, CCMV delta 25, CCMV his and CCMV delta 25 his. The ladder starts at 500 bp ]] | ||
Thursday: | Thursday: | ||
| - | |||
*Prepare the samples for EM and DLS | *Prepare the samples for EM and DLS | ||
*Took pictures with EM | *Took pictures with EM | ||
| - | * | + | *Analyzed the particles with DLS |
| + | <p align="justify"> | ||
| + | Thursday in the morning we prepared the samples for the Electron Microscopy and the Dynamic Light Scattering. Around 10:00 am we traveled to virology for the Electron Microscopy. After taking pictures, we traveled back to get our explanation for the Dynamic Light Scattering machine. We tested three samples; one wild type CCMV, one CCMV-VLP and our own sample. | ||
| + | </p> | ||
| - | |||
| - | + | '''CCMV & delta26 CCMV bricking:''' | |
| - | + | ||
| - | + | ||
| - | '''CCMV & | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Friday: | Friday: | ||
| - | *Started with digestion and ligation into the | + | *Started with digestion and ligation into the linearized backbone |
| - | * | + | *Transformed the ''E.coli'' with our first brick constructs |
| - | * | + | *Grown over the weekend |
| - | + | <p align="justify"> | |
| - | The next day we worked further with the bricking. The PCR products were digested and ligated into our | + | The next day we worked further with the bricking. The PCR products were digested and ligated into our linearized backbones, to form our very first brick. The next thing to do was to transform our ''E.coli'' strain with our bricks. |
| - | We | + | We have to wait now until Monday to see if our transformation is successful. |
| - | + | </p> | |
written by: Mark | written by: Mark | ||
Latest revision as of 00:52, 27 September 2012
week 5: 28 may - 3 june
Office work
This week a lot of new information has been added to the wiki. There is now a team description, the journal has been updated for the most part and a lot of the protocols have been added. We've also worked further with the quaternary structure program, with minor successes. Furthermore the logo is finished and will be uploaded on the wiki next week. Thursday evening we started thinking how to call our project, but the group is still divided. On Friday we worked with the data of the Electron Microscopy and Dynamic Light Scattering and send the results and conclusions to our advisors. Hopefully the results will be posted next week on the wiki.
Wiki page
Munich
Modeling
written by: Mark
[Meeting]
Lab work
CCMV test protocol:
Monday:
- Western Blotting
- prepared 10x Towbins electrotransfer buffer
- sandwich build-up:
- case (white side)
- sponge
- Whatman paper
- Whatman paper
- nitrocellulose membrane
- SDS gel
- Whatman paper
- Whatman paper
- sponge
- case (black side)
- all layers carefully soaked in 1x electrotransfer buffer
- run at 0.15A overnight in 4°C cold room
- missed a secondary antibody, no result available
Tuesday:
- Prepare samples for ultracentrifuge according to the protocol
- Starting ultra centrifuge, 45000 RPM for 3 hours
This was a very productive week, with good results. After the dialysis of last week, We began to prepare the CCMV samples for ultracentrifuge. After the preparation, the ultracentrifuge was started and the three hour wait began. Afterwards We made an appointment for Thursday for the Electron Microscopy and the Dynamic Light Scattering machine.
Wednesday:
- Started second CCMV VLP production
- Started PCR for CCMV wt, CCMV delta 25 variances with pre and suffix
- Started PCR for CCMV his tag and CCMV delta 25 his tag variances with pre and suffix
Thursday:
- Prepare the samples for EM and DLS
- Took pictures with EM
- Analyzed the particles with DLS
Thursday in the morning we prepared the samples for the Electron Microscopy and the Dynamic Light Scattering. Around 10:00 am we traveled to virology for the Electron Microscopy. After taking pictures, we traveled back to get our explanation for the Dynamic Light Scattering machine. We tested three samples; one wild type CCMV, one CCMV-VLP and our own sample.
CCMV & delta26 CCMV bricking:
Friday:
- Started with digestion and ligation into the linearized backbone
- Transformed the E.coli with our first brick constructs
- Grown over the weekend
The next day we worked further with the bricking. The PCR products were digested and ligated into our linearized backbones, to form our very first brick. The next thing to do was to transform our E.coli strain with our bricks. We have to wait now until Monday to see if our transformation is successful.
written by: Mark
 "
"