Team:Evry/Notebook/w10
From 2012.igem.org
(Difference between revisions)
JorgeLindo (Talk | contribs) |
|||
| (28 intermediate revisions not shown) | |||
| Line 52: | Line 52: | ||
pSB1C3/IRES<br> | pSB1C3/IRES<br> | ||
pSB1C3/IaaH-IRES<br> | pSB1C3/IaaH-IRES<br> | ||
| + | -> the result of the colony PCR is not as expected, IRES and IaaH are not present in pSB1C3 | ||
| + | <h2>Thursday, 16th August</h2> | ||
| + | <h3>PCR</h3> | ||
| + | IaaH with primers 34 and 20, IaaM with primers 35 and 22, IRES with primers 31 and 32<br> | ||
| + | Tir I "mega" PCR: with Tir I P1-P4 and TirI P2-P36 PCR products + P1 and P2 primers<br> | ||
| + | <h3>pSC2+</h3> | ||
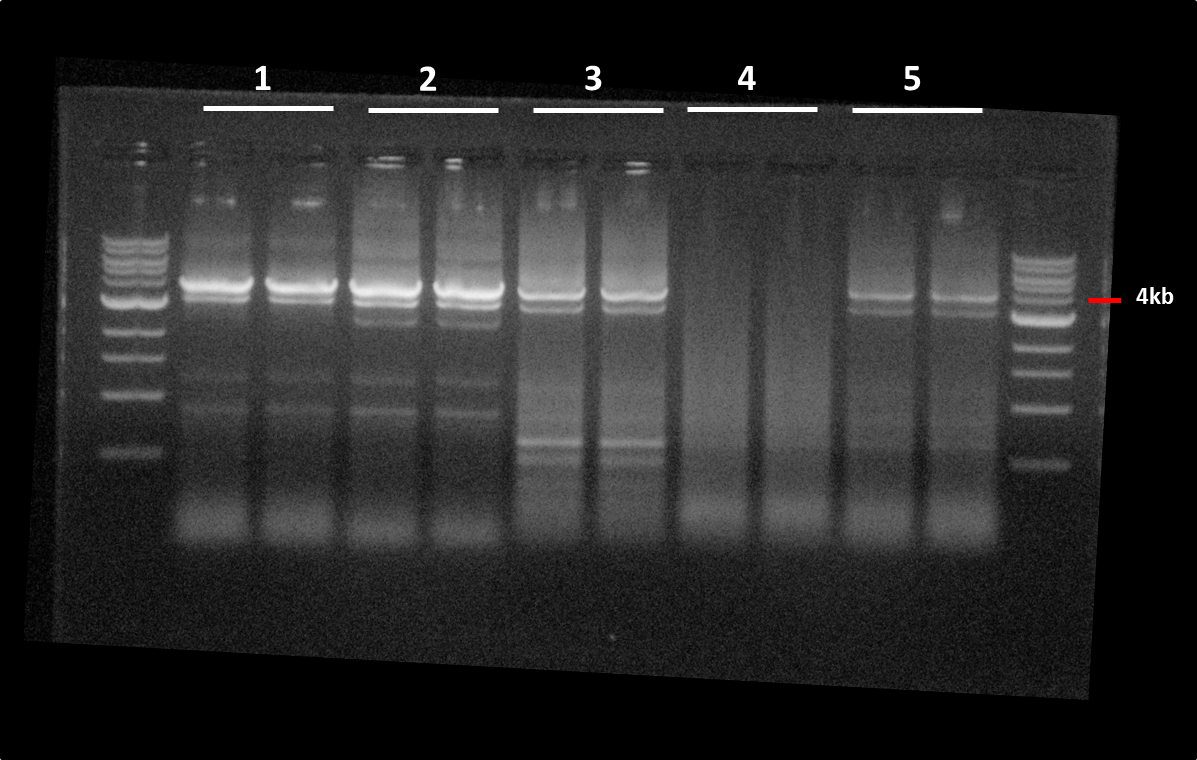
| + | <b>PCR pSC2+</b> samples J5, J6, J18, J21, purif 13/08 with Primers: P7&P8<br> | ||
| + | </html>[[File:GelKC1608.png|left|400px]]<html><br> | ||
| + | (1) PCR sample J5<br> | ||
| + | (2) PCR sample J6<br> | ||
| + | (3) PCR sample J18<br> | ||
| + | (4) PCR sample J21<br> | ||
| + | (5) PCR purif 13/08<br><br><br><br><br><br><br><br><br> | ||
| + | <b>Gel extract</b> 1, 2, 3 and 5. Elution in 20ul in EB<br> | ||
| + | (1) 10,2 ng/ul<br> | ||
| + | (2) 45,6 ng/ul<br> | ||
| + | (3) 20,3 ng/ul<br> | ||
| + | (4) .<br> | ||
| + | (5) 25,6 ng/ul<br><br> | ||
| + | <b>Ligation</b><br> | ||
| + | RFP-Dig16/08-WR: 5ng/ul<br> | ||
| + | IaaH-Purif13/08-KC: 84,5ng/ul <br> | ||
| + | pSC2+-n°5-gelextract-KC: 25,6ng/ul<br><br> | ||
| + | <i>Ligation pSC2+/RFP</i><br> | ||
| + | H2Odd 1,92ul<br> | ||
| + | Buffer 1,5ul<br> | ||
| + | pSC2+ 3,91ul<br> | ||
| + | RFP 6,67ul<br> | ||
| + | T4 ligase 1ul<br><br> | ||
| + | <i>Ligation pSC2+/IaaH</i><br> | ||
| + | H2Odd 7,49ul<br> | ||
| + | Buffer 1,5ul<br> | ||
| + | pSC2+ 3,91ul<br> | ||
| + | IaaH 1,1ul ==> no more material so 0,5ul<br> | ||
| + | T4 ligase 1ul<br><br> | ||
| + | Overnight 16°C<br> | ||
| + | |||
| + | <h3><b> Biobricking Promoters : Flk-1 & CarA</b></h3> | ||
| + | |||
| + | <h2>Friday, 17th August</h2> | ||
| + | <h3>Transformation</h3> | ||
| + | Ligations 16/08<br> | ||
| + | 10ul ligation + 50ul top10 competent cells<br> | ||
| + | 500ul LB pre-warmed<br> | ||
| + | No centrifugation before spread 150ul on plate AMP<br> | ||
| + | Incubation until the end of day at 37°C then RT for the week end<br> | ||
| + | <h3>Gels</h3> | ||
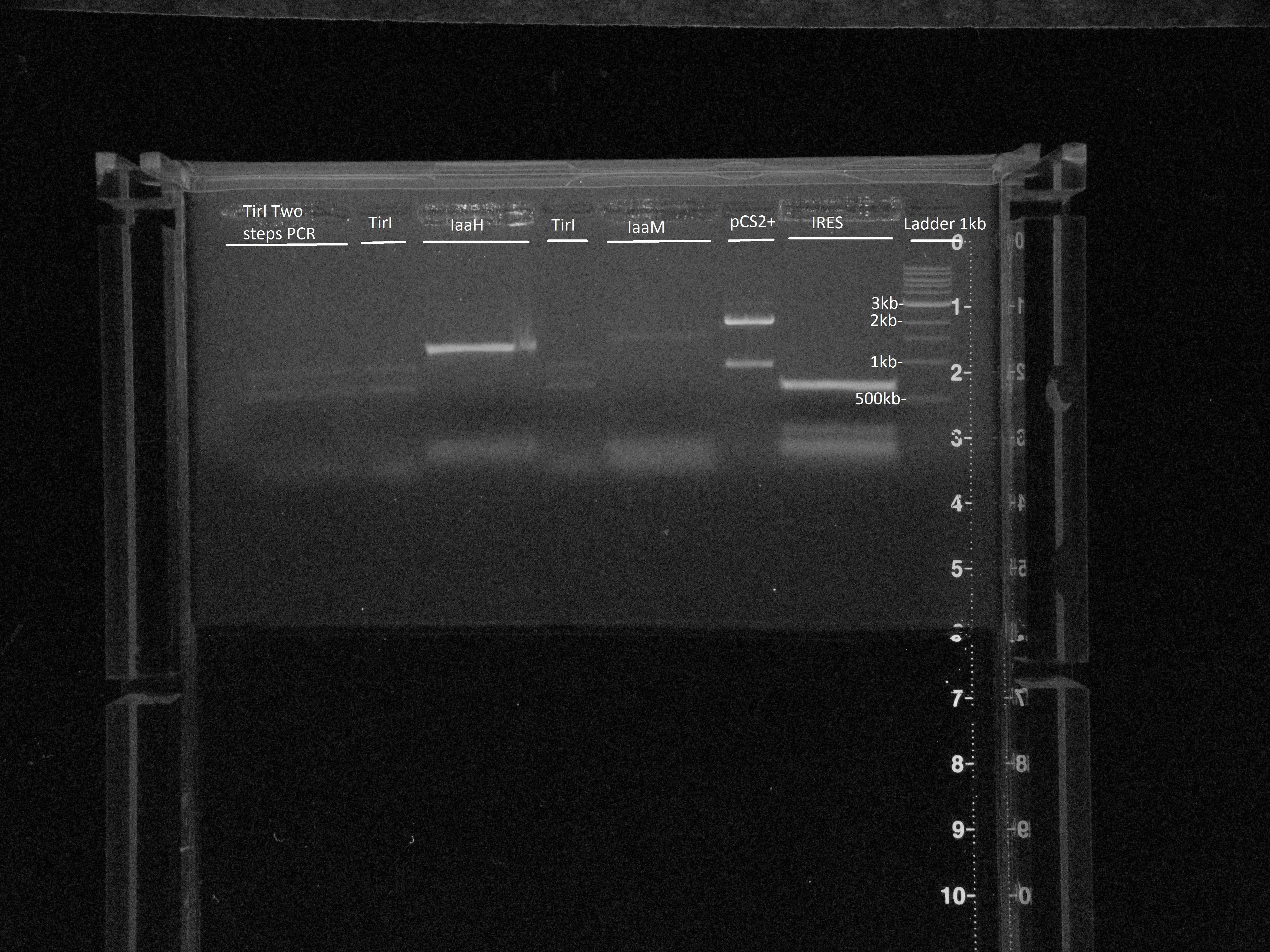
| + | Gel extraction: | ||
</html> | </html> | ||
| + | [[File:Gel_17.08.jpg|center|600px]] | ||
| + | Size is ok for IaaH, IaaM and IRES. | ||
Latest revision as of 13:38, 17 August 2012
Weeks:
| June | July | August | September | October | November | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Week 10: 13th August - 19th August
Monday, 13th August
Tadpoles injection (pNHK60)
Preparation of pNHK60 dilution(phenol/chloro extraction of Friday, 10th August)Tube A12 (50ng/ul): 1,695ul A11 + 73,305ul H2Odd
Tube A13 (25ng/ul): 25ul A12 + 25ul H2Odd
PCR
TirI
Primers: P1 and P2ADN: TirI-PCR P2-P36 (08.08) + TirI PCR P1-P4 (10.08)
pCS2+
Primers: P7+P8ADN:A1
=> These PCR didn't work
New PCR
-TirI + P1-P4 with or without DMSO-TirI + P2-P36 with or without DMSO
-pCS2+ mCherry + P7-P8
-pCS2+ TC + P7-P8
Cloning pSB1C3/IRES, pSB1C3/IaaM, pSB1C3/IaaH, pCS2+/IaaH (to biobrick pCS2+)
Restriction with EcoRI and PstI for pSB1C3 (K515100) then gel extraction of the backboneRestriction with EcoRI and PstI for product of PCR: IRES, IaaH, IaaM and pCS2+ then purification with PCR clean up kit
Then ligation of pCS2+ with IaaH, pSB1C3 with IRES, pSB1C3 with IaaM and pSB1C3 with IaaH
Injection of pNHK60 into Xenopus eggs
Directly after fertilization of xenopus tropicalis eggs: injection of pNHK60 (plasmid with Tir1 and GFP-aid)The production of GFP is expected into eggs
Transformation into Top10 E. coli
10ul sample + 50ul competent cells 250ul SOC medium pSB1C3/IRESpSB1C3/IaaM
pSB1C3/IaaH
pSB1C3/IaaH-IRES
pCS2+/IaaH
pCS2+/IRES
Plates
pSB1C3 chloramphenicol resistantpCS2+ ampicillin resistant
Tuesday, 14th August
Colony PCR
Positive Top 10 colonies:pSB1C3/IRES
pSB1C3/IaaH-IRES
-> the result of the colony PCR is not as expected, IRES and IaaH are not present in pSB1C3
Thursday, 16th August
PCR
IaaH with primers 34 and 20, IaaM with primers 35 and 22, IRES with primers 31 and 32Tir I "mega" PCR: with Tir I P1-P4 and TirI P2-P36 PCR products + P1 and P2 primers
pSC2+
PCR pSC2+ samples J5, J6, J18, J21, purif 13/08 with Primers: P7&P8(1) PCR sample J5
(2) PCR sample J6
(3) PCR sample J18
(4) PCR sample J21
(5) PCR purif 13/08
Gel extract 1, 2, 3 and 5. Elution in 20ul in EB
(1) 10,2 ng/ul
(2) 45,6 ng/ul
(3) 20,3 ng/ul
(4) .
(5) 25,6 ng/ul
Ligation
RFP-Dig16/08-WR: 5ng/ul
IaaH-Purif13/08-KC: 84,5ng/ul
pSC2+-n°5-gelextract-KC: 25,6ng/ul
Ligation pSC2+/RFP
H2Odd 1,92ul
Buffer 1,5ul
pSC2+ 3,91ul
RFP 6,67ul
T4 ligase 1ul
Ligation pSC2+/IaaH
H2Odd 7,49ul
Buffer 1,5ul
pSC2+ 3,91ul
IaaH 1,1ul ==> no more material so 0,5ul
T4 ligase 1ul
Overnight 16°C
Biobricking Promoters : Flk-1 & CarA
Friday, 17th August
Transformation
Ligations 16/0810ul ligation + 50ul top10 competent cells
500ul LB pre-warmed
No centrifugation before spread 150ul on plate AMP
Incubation until the end of day at 37°C then RT for the week end
Gels
Gel extraction:Size is ok for IaaH, IaaM and IRES.
 "
"