Team:Peking/Modeling/Ring
From 2012.igem.org
m |
|||
| (6 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
<html></p></html>{{Template:Peking2012_Color_Prologue}}{{Template:Peking2012_Color_Modeling}}<html> | <html></p></html>{{Template:Peking2012_Color_Prologue}}{{Template:Peking2012_Color_Modeling}}<html> | ||
<script type="text/javascript"> | <script type="text/javascript"> | ||
| - | sublists_Now = | + | sublists_Now = 3; |
| - | var subsubitem=subfirst.getElementsByTagName('ul')[sublists_Now].getElementsByTagName('a')[ | + | var subsubitem=subfirst.getElementsByTagName('ul')[sublists_Now].getElementsByTagName('a')[0]; |
subsubitem.style.color='#60b0f0'; | subsubitem.style.color='#60b0f0'; | ||
listTrigger(sublists_Now); | listTrigger(sublists_Now); | ||
| Line 10: | Line 10: | ||
<h3 id="title1">Background</h3> | <h3 id="title1">Background</h3> | ||
<p> | <p> | ||
| - | Genetic engineering circuits in <i>E.coli<i> enable cells to perform programmably; however, more complex functions are limited by leakage of the gene expression. Consider that cells are able to detect environmental signals such as explosives (e.g., RDX and TNT), toxins, metals, salinity, pH, temperature and light, cell-cell communication-based multicellular networks provide an extended vista for synthetic biology.<br /><br /> | + | Genetic engineering circuits in <i>E.coli</i> enable cells to perform programmably; however, more complex functions are limited by leakage of the gene expression. Consider that cells are able to detect environmental signals such as explosives (e.g., RDX and TNT), toxins, metals, salinity, pH, temperature and light, cell-cell communication-based multicellular networks provide an extended vista for synthetic biology <sup><a href="#ref1" title="Subhayu Basu et al.(2005), A synthetic multicellular system for programmed pattern formation. Nature, vol.434: 1130: 1134">[1]</a></sup>.<br /> |
| - | As a hallmark of coordinated cellular behavior, pattern formation typically required cell-cell communication and intracellular signal processing. For more site-specific signaling and pattern formation, light may be more appropriate alternative. Due to the high sensitivity of our Luminesensor, it is possible to construct a ring-like pattern based on light-communication, previously done by AHL. | + | <br /> |
| + | As a hallmark of coordinated cellular behavior, pattern formation typically required cell-cell communication and intracellular signal processing. For more site-specific signaling and pattern formation, light may be more appropriate alternative. Due to the high sensitivity of our <i>Luminesensor</i>, it is possible to construct a ring-like pattern based on light-communication, previously done by AHL. This kind of flexibility enables its application in tissue-engineering, bio-sensing and bio-manufacturing. | ||
</p> | </p> | ||
</div> | </div> | ||
| Line 17: | Line 18: | ||
<div class="PKU_context floatR first"> | <div class="PKU_context floatR first"> | ||
<h3 id="title1">Circuit Design</h3> | <h3 id="title1">Circuit Design</h3> | ||
| - | + | <p> | |
| + | The high sensitivity of <i>Luminesensor</i> extends its field applications and enables us to achieve light-communication between E. coli cells. As demonstrations, we constructed and ring-like pattern in E. coli based on light-communication between cells. <br /> | ||
| + | <br /> | ||
| + | Figure 1 illustrates the design of the ring-like pattern formation multicellular system. There are two kinds of E. coli cells on the plate <sup><a href="#ref1" title="Subhayu Basu et al.(2005), A synthetic multicellular system for programmed pattern formation. Nature, vol.434: 1130: 1134">[1]</a></sup>: sender cells and receiver cells. Receiver cells are uniformly mixed in the plate medium, while sender cells are place on the surface of the plate, specifically, on the middle point. Sender cells emit light whose wavelength at 460 to 490 nm, where <i>Luminesensor</i> in the receiver cells would be activated and then coordinate cells' behavors. | ||
| + | </p> | ||
| + | <div class="floatC"> | ||
| + | <img src="/wiki/images/9/9a/Band_Circuit_1.jpg" alt="" style="width:500px;"/> | ||
| + | <p class="description"> | ||
| + | Figure 1. Genetic Circuit of Ring Pattern Formation. | ||
| + | </p> | ||
| + | </div> | ||
| + | <p> | ||
| + | Sender cells in the center of the plate produce blue light (490nm) on the addition of arabinose. Then receiver cells around the senders receive the light and react differently to the different light intensity. A high threshold and a low threshold are created in the system, between which the GFP are activated. In the region with high light intensity, both LacIM1 and TetR are repressed; GFP could not be expressed because of the repression by LacI. On the region with low light intensity, GFP could not be expressed either because of the repression by LacIM1. Only on the region with specific light intensity, i.e. the specific distance from the sender, GFP could be expressed. Because the copy number of TetR is set to be more than LacIM1, the light are not enough to shut TetR off, the leaky expression of TetR would repress the repressor of GFP. | ||
| + | </p> | ||
</div> | </div> | ||
| Line 23: | Line 37: | ||
<h3 id="title2">Reference</h3> | <h3 id="title2">Reference</h3> | ||
<p></p> | <p></p> | ||
| - | <ul class="refer"><li id="ref1"> | + | <ul class="refer"> |
| - | 1. | + | <li id="ref1"> |
| - | + | 1. Subhayu Basu et al.(2005), A synthetic multicellular system for programmed pattern formation. <i>Nature</i>, vol.434: 1130: 1134 | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
</li></ul> | </li></ul> | ||
</div> | </div> | ||
</html>{{Template:Peking2012_Color_Epilogue}} | </html>{{Template:Peking2012_Color_Epilogue}} | ||
Latest revision as of 05:06, 26 October 2012
Background
Genetic engineering circuits in E.coli enable cells to perform programmably; however, more complex functions are limited by leakage of the gene expression. Consider that cells are able to detect environmental signals such as explosives (e.g., RDX and TNT), toxins, metals, salinity, pH, temperature and light, cell-cell communication-based multicellular networks provide an extended vista for synthetic biology [1].
As a hallmark of coordinated cellular behavior, pattern formation typically required cell-cell communication and intracellular signal processing. For more site-specific signaling and pattern formation, light may be more appropriate alternative. Due to the high sensitivity of our Luminesensor, it is possible to construct a ring-like pattern based on light-communication, previously done by AHL. This kind of flexibility enables its application in tissue-engineering, bio-sensing and bio-manufacturing.
Circuit Design
The high sensitivity of Luminesensor extends its field applications and enables us to achieve light-communication between E. coli cells. As demonstrations, we constructed and ring-like pattern in E. coli based on light-communication between cells.
Figure 1 illustrates the design of the ring-like pattern formation multicellular system. There are two kinds of E. coli cells on the plate [1]: sender cells and receiver cells. Receiver cells are uniformly mixed in the plate medium, while sender cells are place on the surface of the plate, specifically, on the middle point. Sender cells emit light whose wavelength at 460 to 490 nm, where Luminesensor in the receiver cells would be activated and then coordinate cells' behavors.
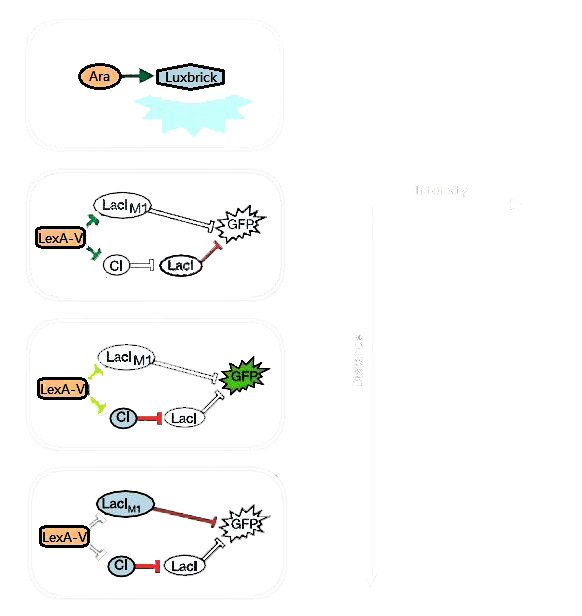
Figure 1. Genetic Circuit of Ring Pattern Formation.
Sender cells in the center of the plate produce blue light (490nm) on the addition of arabinose. Then receiver cells around the senders receive the light and react differently to the different light intensity. A high threshold and a low threshold are created in the system, between which the GFP are activated. In the region with high light intensity, both LacIM1 and TetR are repressed; GFP could not be expressed because of the repression by LacI. On the region with low light intensity, GFP could not be expressed either because of the repression by LacIM1. Only on the region with specific light intensity, i.e. the specific distance from the sender, GFP could be expressed. Because the copy number of TetR is set to be more than LacIM1, the light are not enough to shut TetR off, the leaky expression of TetR would repress the repressor of GFP.
Reference
- 1. Subhayu Basu et al.(2005), A synthetic multicellular system for programmed pattern formation. Nature, vol.434: 1130: 1134
 "
"














