Team:Peking/Modeling
From 2012.igem.org
| (157 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | <html> | + | <html></p></html>{{Template:Peking2012_Color_Prologue}}{{Template:Peking2012_Color_Modeling}}<html> |
| - | < | + | <script type="text/javascript"> |
| - | + | sublists_Now = 0; | |
| - | + | var subsubitem=subfirst.getElementsByTagName('ul')[sublists_Now].getElementsByTagName('a')[0]; | |
| - | + | subsubitem.style.color='#60b0f0'; | |
| - | + | listTrigger(sublists_Now); | |
| - | + | subsubitem.innerHTML=subsubitem.innerHTML+' >>>'; | |
| - | + | </script> | |
| - | + | <div class="PKU_context floatR first"> | |
| - | </ | + | <h3 id="title1">Summary</h3> |
| - | < | + | <p> |
| - | < | + | Our modeling group conducted simulations to guide our experiments by combining protein kinetics, thermodynamics, stochastic simulation, and molecular docking together. Specifically, we conducted modeling of our <i>Luminesensor</i>, and the experimental results of the bio-othorgonality part excellently correspond to the model! We also used molecular docking to expand the spectrum of the <i>Luminesensor</i>, which would greatly extend its application, such as multiple-channel communication among cells. In addition, we simulated the process of phototaxis both on the macro level and micro level, using mean-field PDE (partial difference equations) and Stochastic Simulation respectively. In the PDE model, we developed a hexagonal-coordinate simulation environment for dynamic system on a continuous plane, which would be a very useful prototype for future simulation on cellular movement, pattern formation, and other potential systems. Furthermore, we designed the genetic circuit of multi-cellular ring-like pattern formation as a demonstration for cell-cell communication through light; based on parameter analysis, we used our modeling prediction to rationally guide the experiment to form better patterns! |
| - | < | + | </p> |
| - | + | </div> | |
| - | + | <div class="PKU_context floatR"> | |
| - | + | <h3 id="title2"><i>Luminesensor</i> Modeling</h3> | |
| - | + | <div class="floatR"> | |
| - | < | + | <img src="/wiki/images/3/3a/Peking2012_LuminesensorNodes.png" alt="" style="width:250px;"/> |
| - | + | </div> | |
| - | + | <p> | |
| - | < | + | Our <i>Luminesensor</i> is a ultra-sensitive fusion protein to sense 450nm to 470nm light and then regulate the gene expression(<a href="/Team:Peking/Project/Luminesensor/Future#FigS">spectrum data here</a>). Although the <i>Luminesensor</i> excels and eclipses similar systems due to its ultra-sensitivity and dynamic range, there are still several imperfect aspects. For example, the response time of the protein can be up to hours<sup><a href="#ref1" title="Zoltowski, B.D., Crane, B.R.(2008). Light Activation of the LOV Protein Vivid Generates a Rapidly Exchanging Dimer. Biochemistry, 47: 7012: 7019">[1]</a></sup> and the contrast of binding efficiency with and without light has much room for improvement. After modeling the DNA binding process of the <i>Luminesensor</i>, we managed to find out four key parameters, two of which mainly control the response time, and the others control the contrast of binding efficiency. According to the feasibility in experiment, we tuned two most facile parameters of each aspect to optimize the performance of the <i>Luminsensor</i>, and later discovered mutation sites related to these two parameters experimentally.<br /><br /> |
| - | + | Furthermore, in order to extend the application of the <i>Luminsensor</i>, we managed to expand the spectrum of the <i>Luminsensor</i> through modeling. Here we use molecular modeling to identify the possiblity of modifications to the conjugating substructure of Flavin Adanine Dinucleotide (FAD) substrate, which acts as the chromophore of VVD. | |
| - | </ | + | </p> |
| + | </div> | ||
| + | <div class="PKU_context floatR"> | ||
| + | <h3 id="title3">Phototaxis Modeling</h3> | ||
| + | <div class="floatR"> | ||
| + | <img src="/wiki/images/b/b6/Peking2012_PhototaxisSPECSdot.gif" alt="" style="width:250px;padding:15px 10px 20px 15px;"/> | ||
| + | </div> | ||
| + | <p> | ||
| + | Phototaxis is a light-control bio-system, whose input is the space-distribution of light. By comparison with chemotaxis system, phototaxis has many advantages for application. (See <a href="/Team:Peking/Project/Phototaxis">Project Phototaxis</a>) We have constructed a simple phototaxis system coupling our <i>Luminesensor</i> with the expression level of cheZ protein. In order to confirm the macro light-control to our phototaxis system, we used the Mean-field PDE model. Later we managed to confirm these phenomena by tracing each cells in Stochastic Simulation. We then managed to do the related experiments to prove the effect of light in this simple system. | ||
| + | <br /> <br /> | ||
| + | On the way to the simulation of the macro population changing process, we developed a hexagonal-coordinate simulation environment for dynamic system on a continuous plane. Since the number of neighbors of a chunk unit in the hexagonal mesh is larger than that of the traditional quadratic mesh, the hexagonal mesh is much closer to an isotropic grid, which would reduce the error caused by anisotropic structure in traditional quadratic mesh. This simulation environment would be a useful prototype for future simulation of 2D dynamic systems, such as cellular movement on plate and pattern formation based on cell-cell communication<sup><a href="#ref2" title="Liu, C.(2011). Sequential Establishment of Stripe Patterns in an Expanding Cell Population. <i>Science</i>, 334: 328">[2]</a></sup>. | ||
| + | </p> | ||
| + | </div> | ||
| + | |||
| + | <div class="PKU_context floatR"> | ||
| + | <h3 id="title3">Ring Pattern Formation</h3> | ||
| + | <div class="floatR"> | ||
| + | <img src="/wiki/images/c/cf/Band_Origin_3.png" alt="" style="width:250px;padding:15px 10px 20px 15px;"/> | ||
| + | </div> | ||
| + | <p> | ||
| + | As a hallmark of coordinated cellular behavior, pattern formation typically required cell-cell communication and intracellular signal processing. For more site-specific signaling and pattern formation, light may be more appropriate alternative. Due to the high sensitivity of our <i>Luminesensor</i>, it is possible to construct a ring-like pattern based on light-communication, previously done by AHL. This kind of flexibility enables its application in tissue-engineering, bio-sensing and bio-manufacturing. | ||
| + | <br /> <br /> | ||
| + | The high sensitivity of <i>Luminesensor</i> extends its field applications and enables us to achieve light-communication between E. coli cells. As demonstrations, we constructed and ring-like pattern in E. coli based on light-communication between cells. There are two kinds of E. coli cells on the plate: sender cells and receiver cells. Sender cells in the center of the plate produce blue light (490nm) on the addition of arabinose. Then receiver cells around the senders receive the light and react differently to the different light intensity. A high threshold and a low threshold are created in the system, between which the GFP are activated <sup><a href="#ref1" title="Subhayu Basu et al.(2005), A synthetic multicellular system for programmed pattern formation. Nature, vol.434: 1130: 1134">[3]</a></sup>. | ||
| + | </p> | ||
| + | </div> | ||
| + | |||
| + | <div class="PKU_context floatR"> | ||
| + | <h3 id="title5">Reference</h3> | ||
| + | <p></p> | ||
| + | <ul class="refer"><li id="ref1"> | ||
| + | 1. Zoltowski, B.D., Crane, B.R.(2008). Light Activation of the LOV Protein Vivid Generates a Rapidly Exchanging Dimer. <i>Biochemistry</i>, 47: 7012-7019 | ||
<br /> | <br /> | ||
| + | 2. Liu, C. <i>et al</i> (2011). Sequential Establishment of Stripe Patterns in an Expanding Cell Population. <i>Science</i>, 334: 328 | ||
<br /> | <br /> | ||
| - | < | + | 3. Subhayu Basu et al.(2005), A synthetic multicellular system for programmed pattern formation. <i>Nature</i>, vol.434: 1130: 1134 |
| - | < | + | </li></ul> |
| - | < | + | </div> |
| - | < | + | </html>{{Template:Peking2012_Color_Epilogue}} |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | </ | + | |
| - | </html> | + | |
Latest revision as of 19:13, 26 October 2012
Summary
Our modeling group conducted simulations to guide our experiments by combining protein kinetics, thermodynamics, stochastic simulation, and molecular docking together. Specifically, we conducted modeling of our Luminesensor, and the experimental results of the bio-othorgonality part excellently correspond to the model! We also used molecular docking to expand the spectrum of the Luminesensor, which would greatly extend its application, such as multiple-channel communication among cells. In addition, we simulated the process of phototaxis both on the macro level and micro level, using mean-field PDE (partial difference equations) and Stochastic Simulation respectively. In the PDE model, we developed a hexagonal-coordinate simulation environment for dynamic system on a continuous plane, which would be a very useful prototype for future simulation on cellular movement, pattern formation, and other potential systems. Furthermore, we designed the genetic circuit of multi-cellular ring-like pattern formation as a demonstration for cell-cell communication through light; based on parameter analysis, we used our modeling prediction to rationally guide the experiment to form better patterns!
Luminesensor Modeling
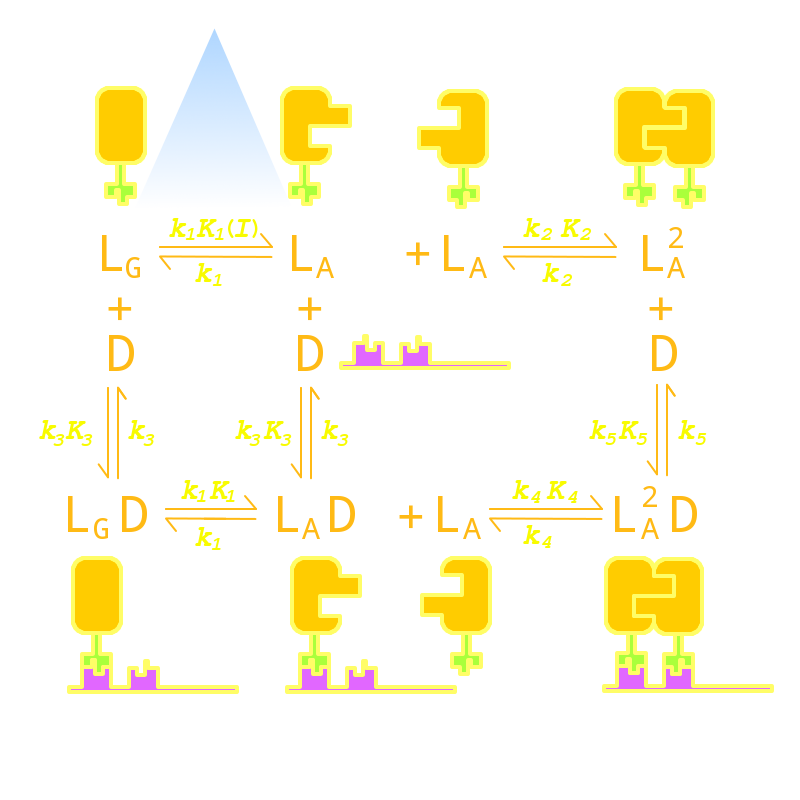
Our Luminesensor is a ultra-sensitive fusion protein to sense 450nm to 470nm light and then regulate the gene expression(spectrum data here). Although the Luminesensor excels and eclipses similar systems due to its ultra-sensitivity and dynamic range, there are still several imperfect aspects. For example, the response time of the protein can be up to hours[1] and the contrast of binding efficiency with and without light has much room for improvement. After modeling the DNA binding process of the Luminesensor, we managed to find out four key parameters, two of which mainly control the response time, and the others control the contrast of binding efficiency. According to the feasibility in experiment, we tuned two most facile parameters of each aspect to optimize the performance of the Luminsensor, and later discovered mutation sites related to these two parameters experimentally.
Furthermore, in order to extend the application of the Luminsensor, we managed to expand the spectrum of the Luminsensor through modeling. Here we use molecular modeling to identify the possiblity of modifications to the conjugating substructure of Flavin Adanine Dinucleotide (FAD) substrate, which acts as the chromophore of VVD.
Phototaxis Modeling
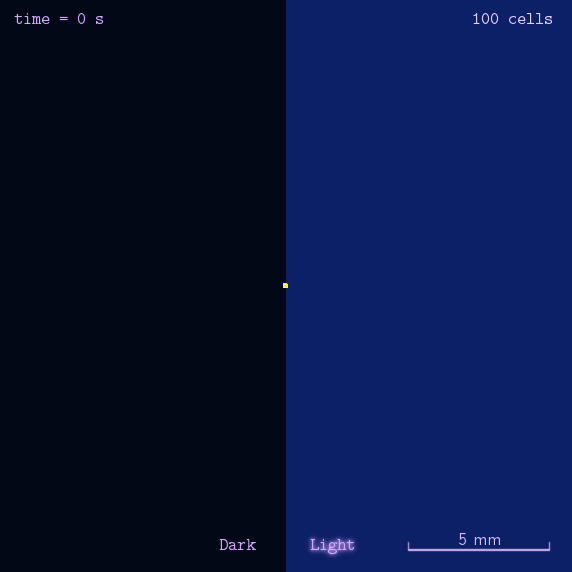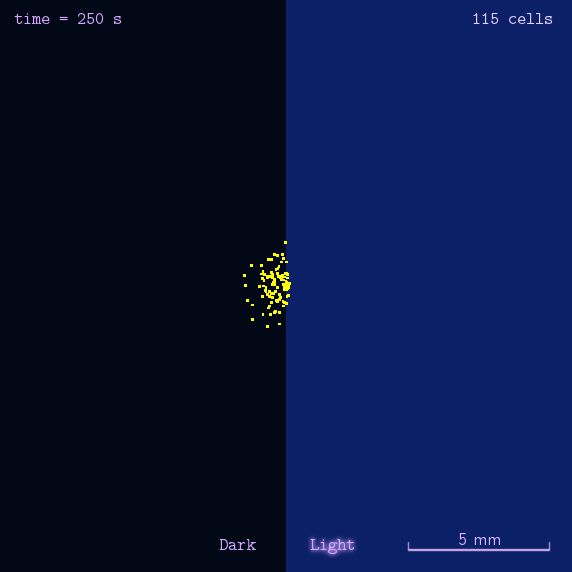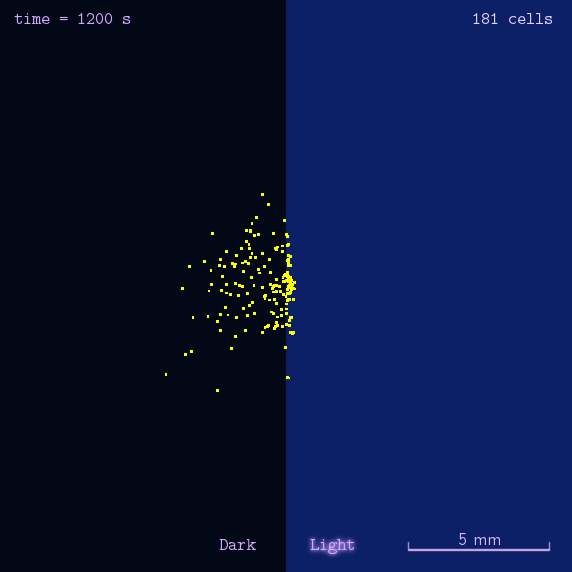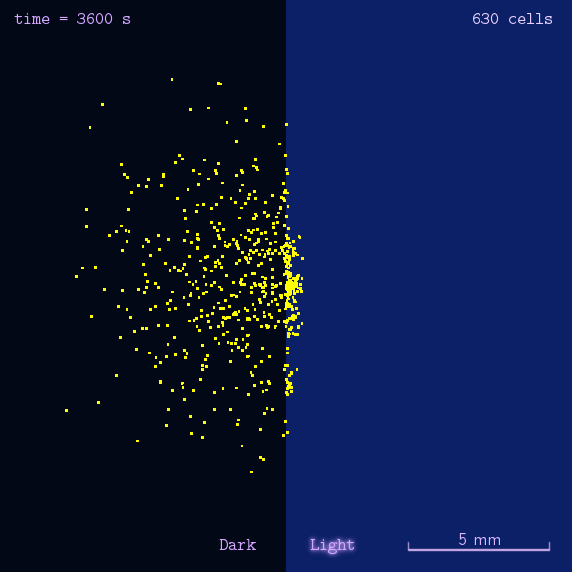
Phototaxis is a light-control bio-system, whose input is the space-distribution of light. By comparison with chemotaxis system, phototaxis has many advantages for application. (See Project Phototaxis) We have constructed a simple phototaxis system coupling our Luminesensor with the expression level of cheZ protein. In order to confirm the macro light-control to our phototaxis system, we used the Mean-field PDE model. Later we managed to confirm these phenomena by tracing each cells in Stochastic Simulation. We then managed to do the related experiments to prove the effect of light in this simple system.
On the way to the simulation of the macro population changing process, we developed a hexagonal-coordinate simulation environment for dynamic system on a continuous plane. Since the number of neighbors of a chunk unit in the hexagonal mesh is larger than that of the traditional quadratic mesh, the hexagonal mesh is much closer to an isotropic grid, which would reduce the error caused by anisotropic structure in traditional quadratic mesh. This simulation environment would be a useful prototype for future simulation of 2D dynamic systems, such as cellular movement on plate and pattern formation based on cell-cell communication[2].
Ring Pattern Formation
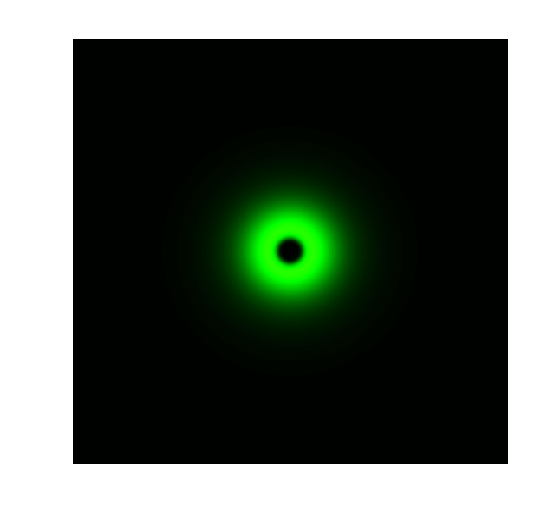
As a hallmark of coordinated cellular behavior, pattern formation typically required cell-cell communication and intracellular signal processing. For more site-specific signaling and pattern formation, light may be more appropriate alternative. Due to the high sensitivity of our Luminesensor, it is possible to construct a ring-like pattern based on light-communication, previously done by AHL. This kind of flexibility enables its application in tissue-engineering, bio-sensing and bio-manufacturing.
The high sensitivity of Luminesensor extends its field applications and enables us to achieve light-communication between E. coli cells. As demonstrations, we constructed and ring-like pattern in E. coli based on light-communication between cells. There are two kinds of E. coli cells on the plate: sender cells and receiver cells. Sender cells in the center of the plate produce blue light (490nm) on the addition of arabinose. Then receiver cells around the senders receive the light and react differently to the different light intensity. A high threshold and a low threshold are created in the system, between which the GFP are activated [3].
Reference
-
1. Zoltowski, B.D., Crane, B.R.(2008). Light Activation of the LOV Protein Vivid Generates a Rapidly Exchanging Dimer. Biochemistry, 47: 7012-7019
2. Liu, C. et al (2011). Sequential Establishment of Stripe Patterns in an Expanding Cell Population. Science, 334: 328
3. Subhayu Basu et al.(2005), A synthetic multicellular system for programmed pattern formation. Nature, vol.434: 1130: 1134
 "
"














