Team:Cambridge/Lab book/Week 7
From 2012.igem.org
(Difference between revisions)
(→Tuesday) |
|||
| Line 10: | Line 10: | ||
|} | |} | ||
| - | ===Monday=== | + | ===Monday (06/08/12)==== |
'''[[Team:Cambridge/Protocols/PCRProtocol|PCR of Magnesium riboswitch vector fragment B and Magnesium promoter]]''' | '''[[Team:Cambridge/Protocols/PCRProtocol|PCR of Magnesium riboswitch vector fragment B and Magnesium promoter]]''' | ||
| Line 32: | Line 32: | ||
*Positive control also failed, although this has ceased to work for several days, potentially due to DNA degradation. | *Positive control also failed, although this has ceased to work for several days, potentially due to DNA degradation. | ||
| - | ===Tuesday=== | + | ===Tuesday (07/08/12)==== |
'''[[Team:Cambridge/Protocols/Electrocompetentcells|Production of electrocompetent e.coli]]''' | '''[[Team:Cambridge/Protocols/Electrocompetentcells|Production of electrocompetent e.coli]]''' | ||
| Line 58: | Line 58: | ||
::*Reaction 3: Tube 4 (07/08/12) (fragment B), Tube 1 (05/08/12) (fragment A), Tube 2 (18/07/12) (riboswitch DNA). | ::*Reaction 3: Tube 4 (07/08/12) (fragment B), Tube 1 (05/08/12) (fragment A), Tube 2 (18/07/12) (riboswitch DNA). | ||
| - | ===Wednesday=== | + | ===Wednesday (08/08/12)=== |
===Thursday=== | ===Thursday=== | ||
Revision as of 09:13, 8 August 2012
| Week: | 3 | 4 | 5 | 6 | 7 |
|---|
Contents |
Monday (06/08/12)=
PCR of Magnesium riboswitch vector fragment B and Magnesium promoter
- Normal PCR settings used, annealing temperature 57 °C, elongation step 90s long.
- Lane 5 accidentally loaded with a DNA ladder instead of loading dye.
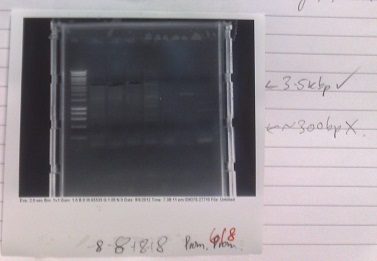
- Expected fragment sizes:
- Lane 2-5: 3kbp
- Lane 6-7: 300bp
- After electrophoresis, found vector products had, for the most part, worked. Promoter elements were not amplified - no band of the expected size was observed.
- Positive control also failed, although this has ceased to work for several days, potentially due to DNA degradation.
Tuesday (07/08/12)=
Production of electrocompetent e.coli
Gibson assembly of magnesium riboswitch and fluorescent construct
- NAD+ added to isothermal buffer*5 mix
- Gel slices from yesterday (of vector fragment B) purified.
- DNA added as follows:
- Without 8 codon substitution:
- Reaction 1: Tube 2 (07/08/12) (fragment B), Tube 1 (05/08/12) (fragment A), Tube 28 (29/08/12) (riboswitch DNA).
- Reaction 2: Tube 3 (07/08/12) (fragment B), Tube 2 (05/08/12) (fragment A), Tube 29 (29/08/12) (riboswitch DNA).
- With 8 codon substitution:
- Reaction 3: Tube 4 (07/08/12) (fragment B), Tube 1 (05/08/12) (fragment A), Tube 2 (18/07/12) (riboswitch DNA).
Wednesday (08/08/12)
Thursday
Friday
 "
"