Team:Osaka/Tests
From 2012.igem.org
(→Promoter assay) |
|||
| (102 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
== Tests == | == Tests == | ||
=== Damage tolerance assay === | === Damage tolerance assay === | ||
| - | To measure the DNA damage tolerance conferred by each part, we | + | Radioresistance parts contain codon rarely used in <i>''E.coli''</i>. Codon optimization and a better expression system are needed to make our parts functional, so we transformed plasmid DNA into <i>''E.coli''</i> Rosetta. |
| - | + | <br> | |
| + | To measure the DNA damage tolerance conferred by each part, we measured the survival rate in the presence of some DNA damaging agents (such as Mitomycin C and Hydrogen peroxide). Transformants ''E. coli'' were exposed to the DNA damaging agents and then incubated for 2 hours. Then, the cells were spreaded on agar plates at different dilutions. The plates were wrapped with aluminum foil and incubated in the dark. Colony-forming units were scored after 16h incubation at 37°C. For detailed protocols, refer to the [https://2012.igem.org/Team:Osaka/Protocols Protocols page]. | ||
| + | The tolerance parts tested were as follows: | ||
=====Parts containing one gene each===== | =====Parts containing one gene each===== | ||
| - | [[File: | + | [[File:CDS1.png|280px]] |
| - | *CDS: PprI, PprA, PprM or RecA | + | *CDS: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K602005 PprI], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K602006 PprA], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K602007 PprM] or [http://partsregistry.org/wiki/index.php?title=Part:BBa_K602008 RecA] |
=====Parts containing two genes===== | =====Parts containing two genes===== | ||
| - | [[File: | + | [[File:CDS2.png|560px]] |
| - | *CDS1+2: PprI+RecA, PprA+RecA, PprM+RecA, PprI+PprA, PprI+PprM, PprA+PprM | + | *CDS1+2: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K602016 PprI+RecA], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K602017 PprA+RecA], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K602020 PprM+RecA], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K602015 PprI+PprA],[http://partsregistry.org/wiki/index.php?title=Part:BBa_K602018 PprI+PprM], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K602019 PprA+PprM], |
| + | |||
| + | |||
| + | [[File:Viability_after_exposure_to_Mitomycin_C.png|800px]] | ||
| + | |||
| + | |||
| + | [[File:Viability after exposure to Hydrogen peroxide.png|800px]] | ||
| + | |||
| + | ==== Discussion ==== | ||
| + | ===== Single-gene parts ===== | ||
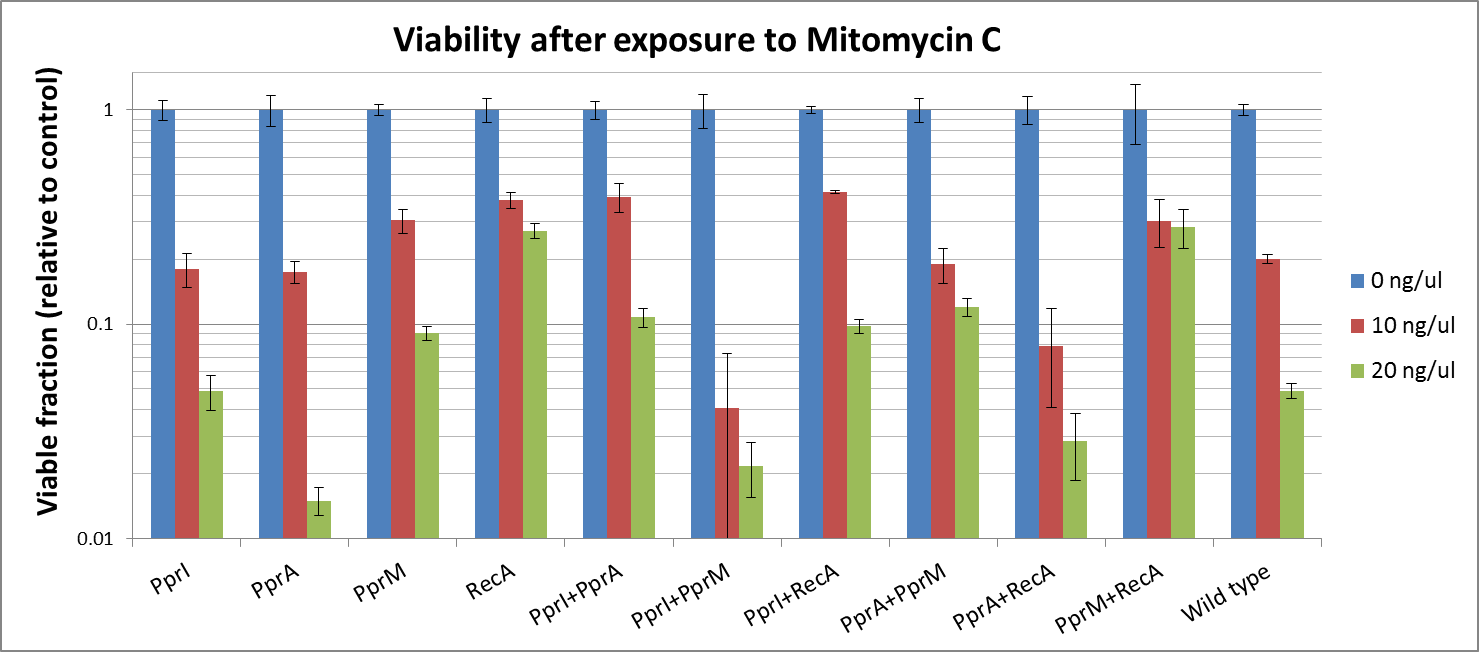
| + | Against Mitomycine C | ||
| + | |||
| + | We found that | ||
| + | * PprI did not increase the tolerance alone. PprI is a pleiotropic gene, which senses DNA lesions, regulates the downstream repair genes and also carries catalase activity. As <i>''E. coli''</i> lacks downstream proteins of <i>D. radiodurans</i>, it is expected that PprI would not be able to confer tolerance on its own. | ||
| + | * PprA, which repairs blunt-ended breaks in DNA lesions, also did not confer tolerance. PprA may not protect against interstrand crosslinks and single strand breaks induced by Mitomycin C. | ||
| + | * In our previous experiment using UV irradiation, PprM showed to confer tolerance slightly. Though it is said that PprM is a modulator of the PprI-dependent damage response that depends on downstream effector such as PprA, our results indicates that it is protective for <i>''E.coli''</i> by itself. | ||
| + | * In our previous experiments, RecA of <i>''D. radiodurans''</i> conferred the highest tolerance among the four radiotolerance genes (against UV irradiation). We showed that RecA much increased tolerance against DNA damaging agents, too. Maybe, it supports the effect of <i>''E.coli''</i>'s native RecA each other. | ||
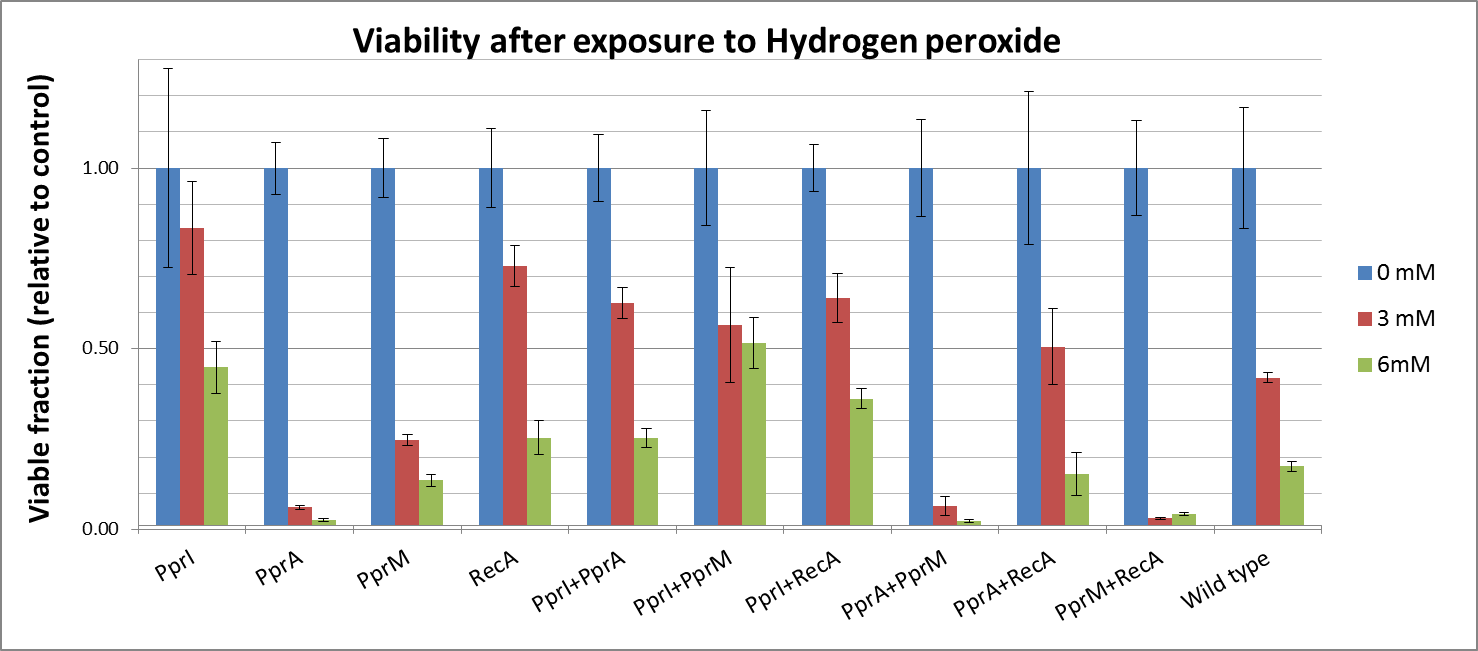
| + | Against Hydrogen peroxide | ||
| + | * Actually, each PprA and PprM alone decreased tolerance. It may be due to the cost of their expression in the stressful cellular environment at least. | ||
| + | * On the other hand, both PprI and RecA significantly increased tolerance, which agrees with the role of PprI as an enhancer of enzyme activities of catalases | ||
| + | |||
| + | ===== Two-gene combinations ===== | ||
| + | Against Mitomycine C | ||
| + | *While PprI alone was unable to confer the tolerance to this agent, the combination of PprI and PprA somehow increased the tolerance. This is in accordance with the role of PprI as an inducer of PprA. | ||
| + | *The fact that this combination much increased the tolerance, agrees with the role of PprI as an inducer of <i>D. radiodurans</i> RecA. | ||
| + | * Though it is said that PprM is not a modulator of RecA, here we shiowed a significant increase in tolerance when these two genes are coupled. It could be explained by the fact that PprM is known to induce/modulate other, unknown proteins and some of these proteins may have homologs in <i>E. coli</i> that benefit from the presence of PprM. | ||
| + | |||
| + | Against Hydrogen peroxide | ||
| + | * Though PprA or PprM alone did not increase tolerance, the combination of PprI and other radiotolerance genes somehow improved the tolerance. This agrees with the role of PprI as an enhancer of enzyme activities of catalases. | ||
| + | |||
| + | ===== Conclusion ===== | ||
| + | In order to render <i>''E. coli''</i> tolrerant to DNA lesions, we tested possible exogenous radioresistance genes originally isolated from <i>''D.radiodurance''</i>. | ||
| + | First, PprM appears to protect against to interstrand crosslinks and/or single strand breaks induced by Mitomycin C. Perhaps its role as a mere modulator of the PprI-dependent DNA damage response needs to be revised, or perhaps it is capable of regulating certain <i>E. coli</i> genes to advantageous effect. | ||
| + | <br> | ||
| + | In addition, our results indicated that PprI, as a global regulator of the DNA repair system, alone does not secure against Mitomycin C and UV irradiation. This is in contradiction to a previous report that PprI confers radiotolerance to <i>''E. coli''</i>. | ||
| + | <br> | ||
| + | Finally, <i>''D. radiodurans''</i>'s RecA confer resistance to any mutagen despite past reports that this protein is lethal to <i>E. coli</i>. | ||
| - | = | + | === Promoter assay === |
| + | [[File:Construction.png|300px]] | ||
| + | <p> | ||
| + | This is our goal of construct to detect and report the DNA damages. This construct needs responsive promoter to sense DNA damage and also needs reporter genes to produce a sort of color pigments. | ||
| + | <br> | ||
| + | Last year, we assayed the promoter of the SOS gene RecA ([http://partsregistry.org/wiki/index.php?title=Part:BBa_J22106 J22106]) by attaching a lycopene biosynthesis gene cluster ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K274100 K274100]) downstream as a reporter. This construct successfully produce lycopene in response to DNA damage. However, it remains unclear in the quantitative relation between promoter activity and color intensity. In order to evaluate the promoter activity quantitatively and accurately, we also employed the Dual Luciferase Reporter Assay System as a control. Transformed ''E. coli'' was exposed to DNA damaging agents and then incubated for 2 hours. For details check the [https://2012.igem.org/Team:Osaka/Protocols Protocols page]. | ||
| + | </p> | ||
| - | == | + | <html> |
| - | + | <font size="3" color="#ff0000"> | |
| + | We are trying to construct this promoter evaluation device utilizing firefly and renilla luciferase, but not yet finished. | ||
| + | </font> | ||
| + | </html> | ||
| - | = | + | === Future work === |
| + | ===== 1, Complete the Dual Luciferase Assay System ===== | ||
| + | <p>We are trying to assemble the Dual Luciferase Assay System from existing Biobrick parts, but have not finished it yet. So, we will go on to complete it. </p> | ||
| + | ===== 2, Quantification of damages to "Bio-dosimeter" ===== | ||
| + | <p>One of the weak point of our "Bio-dosimeter" is difficulty to quantify the damages, so that, to quantify radiation. Therefore, for practical use, we have to evaluate the ovserved output and compare the dosed danage source to quantify the measurement by the "Bio-dosimeter".</p> | ||
Latest revision as of 14:51, 21 October 2012
Tests
Damage tolerance assay
Radioresistance parts contain codon rarely used in E.coli. Codon optimization and a better expression system are needed to make our parts functional, so we transformed plasmid DNA into E.coli Rosetta.
To measure the DNA damage tolerance conferred by each part, we measured the survival rate in the presence of some DNA damaging agents (such as Mitomycin C and Hydrogen peroxide). Transformants E. coli were exposed to the DNA damaging agents and then incubated for 2 hours. Then, the cells were spreaded on agar plates at different dilutions. The plates were wrapped with aluminum foil and incubated in the dark. Colony-forming units were scored after 16h incubation at 37°C. For detailed protocols, refer to the Protocols page.
The tolerance parts tested were as follows:
Parts containing one gene each
- CDS: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K602005 PprI], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K602006 PprA], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K602007 PprM] or [http://partsregistry.org/wiki/index.php?title=Part:BBa_K602008 RecA]
Parts containing two genes
- CDS1+2: [http://partsregistry.org/wiki/index.php?title=Part:BBa_K602016 PprI+RecA], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K602017 PprA+RecA], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K602020 PprM+RecA], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K602015 PprI+PprA],[http://partsregistry.org/wiki/index.php?title=Part:BBa_K602018 PprI+PprM], [http://partsregistry.org/wiki/index.php?title=Part:BBa_K602019 PprA+PprM],
Discussion
Single-gene parts
Against Mitomycine C
We found that
- PprI did not increase the tolerance alone. PprI is a pleiotropic gene, which senses DNA lesions, regulates the downstream repair genes and also carries catalase activity. As E. coli lacks downstream proteins of D. radiodurans, it is expected that PprI would not be able to confer tolerance on its own.
- PprA, which repairs blunt-ended breaks in DNA lesions, also did not confer tolerance. PprA may not protect against interstrand crosslinks and single strand breaks induced by Mitomycin C.
- In our previous experiment using UV irradiation, PprM showed to confer tolerance slightly. Though it is said that PprM is a modulator of the PprI-dependent damage response that depends on downstream effector such as PprA, our results indicates that it is protective for E.coli by itself.
- In our previous experiments, RecA of D. radiodurans conferred the highest tolerance among the four radiotolerance genes (against UV irradiation). We showed that RecA much increased tolerance against DNA damaging agents, too. Maybe, it supports the effect of E.coli's native RecA each other.
Against Hydrogen peroxide
- Actually, each PprA and PprM alone decreased tolerance. It may be due to the cost of their expression in the stressful cellular environment at least.
- On the other hand, both PprI and RecA significantly increased tolerance, which agrees with the role of PprI as an enhancer of enzyme activities of catalases
Two-gene combinations
Against Mitomycine C
- While PprI alone was unable to confer the tolerance to this agent, the combination of PprI and PprA somehow increased the tolerance. This is in accordance with the role of PprI as an inducer of PprA.
- The fact that this combination much increased the tolerance, agrees with the role of PprI as an inducer of D. radiodurans RecA.
- Though it is said that PprM is not a modulator of RecA, here we shiowed a significant increase in tolerance when these two genes are coupled. It could be explained by the fact that PprM is known to induce/modulate other, unknown proteins and some of these proteins may have homologs in E. coli that benefit from the presence of PprM.
Against Hydrogen peroxide
- Though PprA or PprM alone did not increase tolerance, the combination of PprI and other radiotolerance genes somehow improved the tolerance. This agrees with the role of PprI as an enhancer of enzyme activities of catalases.
Conclusion
In order to render E. coli tolrerant to DNA lesions, we tested possible exogenous radioresistance genes originally isolated from D.radiodurance.
First, PprM appears to protect against to interstrand crosslinks and/or single strand breaks induced by Mitomycin C. Perhaps its role as a mere modulator of the PprI-dependent DNA damage response needs to be revised, or perhaps it is capable of regulating certain E. coli genes to advantageous effect.
In addition, our results indicated that PprI, as a global regulator of the DNA repair system, alone does not secure against Mitomycin C and UV irradiation. This is in contradiction to a previous report that PprI confers radiotolerance to E. coli.
Finally, D. radiodurans's RecA confer resistance to any mutagen despite past reports that this protein is lethal to E. coli.
Promoter assay
This is our goal of construct to detect and report the DNA damages. This construct needs responsive promoter to sense DNA damage and also needs reporter genes to produce a sort of color pigments.
Last year, we assayed the promoter of the SOS gene RecA ([http://partsregistry.org/wiki/index.php?title=Part:BBa_J22106 J22106]) by attaching a lycopene biosynthesis gene cluster ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K274100 K274100]) downstream as a reporter. This construct successfully produce lycopene in response to DNA damage. However, it remains unclear in the quantitative relation between promoter activity and color intensity. In order to evaluate the promoter activity quantitatively and accurately, we also employed the Dual Luciferase Reporter Assay System as a control. Transformed E. coli was exposed to DNA damaging agents and then incubated for 2 hours. For details check the Protocols page.
We are trying to construct this promoter evaluation device utilizing firefly and renilla luciferase, but not yet finished.
Future work
1, Complete the Dual Luciferase Assay System
We are trying to assemble the Dual Luciferase Assay System from existing Biobrick parts, but have not finished it yet. So, we will go on to complete it.
2, Quantification of damages to "Bio-dosimeter"
One of the weak point of our "Bio-dosimeter" is difficulty to quantify the damages, so that, to quantify radiation. Therefore, for practical use, we have to evaluate the ovserved output and compare the dosed danage source to quantify the measurement by the "Bio-dosimeter".
 "
"