Team:NTNU Trondheim/Model
From 2012.igem.org
(→Overview) |
(→Modelling for RHIT) |
||
| (21 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:NTNU_Trondheim/Templates/Header}} | {{:Team:NTNU_Trondheim/Templates/Header}} | ||
| + | <html> | ||
| + | <div class="container"> | ||
| + | <div class="page-header-top"> | ||
| + | <h1>Modelling <small>Stochastic simulations of the genetic circuit</small></h1> | ||
| + | </div> | ||
| + | </div> | ||
| - | |||
| + | <div class="container main-container"> | ||
| + | </html> | ||
__TOC__ | __TOC__ | ||
| - | |||
==Overview== | ==Overview== | ||
| Line 19: | Line 25: | ||
All the equations in the model assumed mass action. For the details and parameters used in the model, see [[Team:NTNU_Trondheim/Equations|Equations]]. | All the equations in the model assumed mass action. For the details and parameters used in the model, see [[Team:NTNU_Trondheim/Equations|Equations]]. | ||
| - | A .zip file of the full model can be downloaded [[ | + | A .zip file of the full model can be downloaded [[Media:NTNU_Trondheim_Full.zip|here]]. |
==Lld promoter== | ==Lld promoter== | ||
| Line 30: | Line 36: | ||
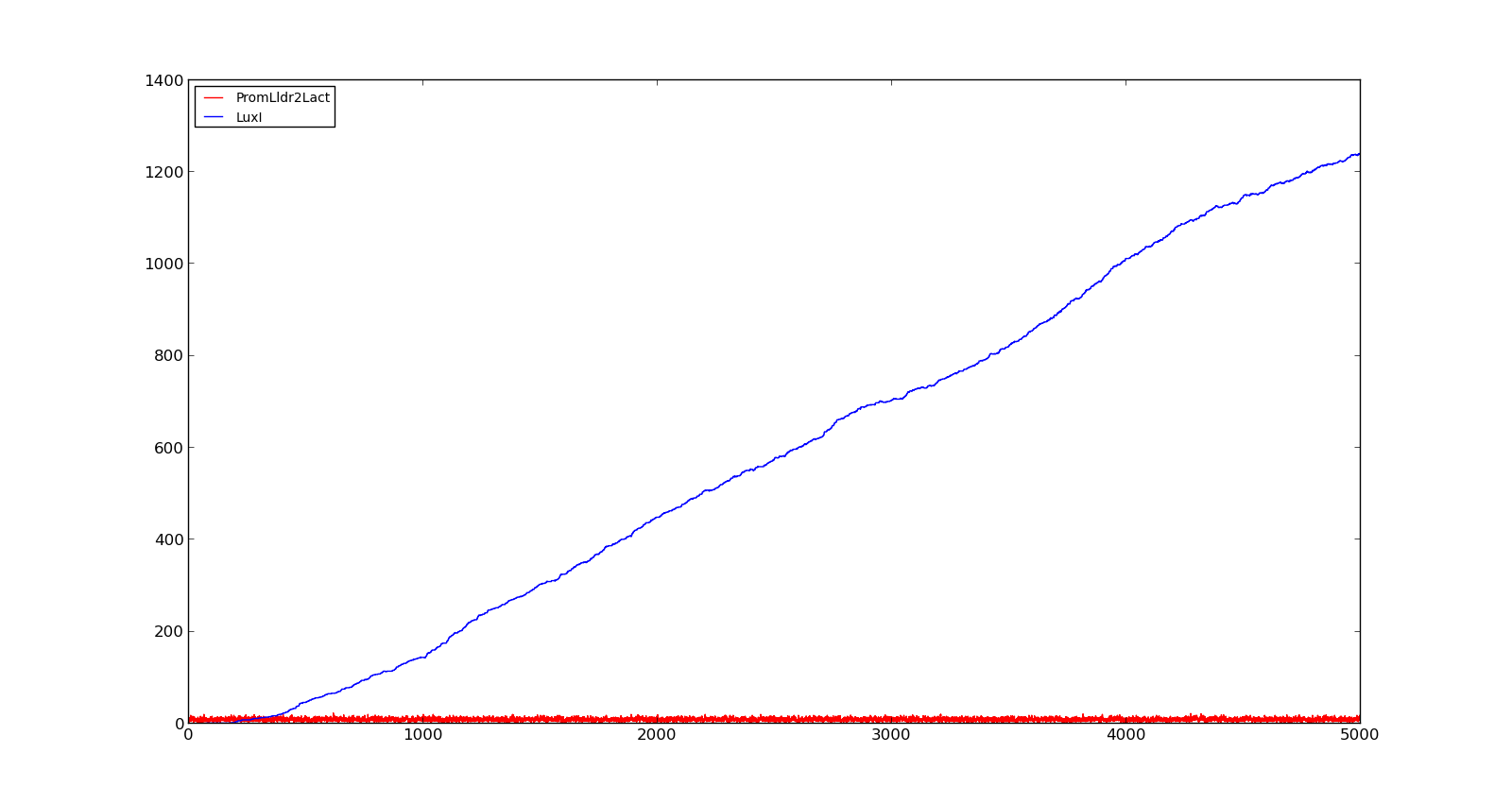
The plots show the production of the LuxI protein as a response to the presence of lactate. The plot on the left shows a system with a quite low concentration of lactate, 10 in the model. Under these conditions, only a few, less than 5, promoters are activated and, as result, the LuxI concentration remains low.The plot on the right shows the response to a 100 times larger lactate concentration. Even though the number of active promoters remains relatively low, the constant production of mRNA leads to a large production of LuxI. | The plots show the production of the LuxI protein as a response to the presence of lactate. The plot on the left shows a system with a quite low concentration of lactate, 10 in the model. Under these conditions, only a few, less than 5, promoters are activated and, as result, the LuxI concentration remains low.The plot on the right shows the response to a 100 times larger lactate concentration. Even though the number of active promoters remains relatively low, the constant production of mRNA leads to a large production of LuxI. | ||
| - | {| style="text-align:center;" | + | {| style="text-align:center; margin: 1em auto 1em auto; padding: 0 10px;" cellpadding="10px" |
|[[File:NTNU_Trondheim_Model_LldrLow.png|thumb|center|450px|Figure 1. Response of lld promoter to a lactate level of 10]] | |[[File:NTNU_Trondheim_Model_LldrLow.png|thumb|center|450px|Figure 1. Response of lld promoter to a lactate level of 10]] | ||
|[[File:NTNU_Trondheim_Model_LldrHigh.png|thumb|center|450px|Figure 2. Response of lld promoter to a lactate level of 1000]] | |[[File:NTNU_Trondheim_Model_LldrHigh.png|thumb|center|450px|Figure 2. Response of lld promoter to a lactate level of 1000]] | ||
| Line 44: | Line 50: | ||
In the figures below, the LuxR production and amount of active promoter is plotted for three different oxygen levels; 10, 250 and 2000. For the levels with low induction, the amount of protein remains relatively low around 100. In the high induction case, however, the concentration rapidly increases to over 900. Even for the high induction case, the amount of activated promoter remains relatively low. | In the figures below, the LuxR production and amount of active promoter is plotted for three different oxygen levels; 10, 250 and 2000. For the levels with low induction, the amount of protein remains relatively low around 100. In the high induction case, however, the concentration rapidly increases to over 900. Even for the high induction case, the amount of activated promoter remains relatively low. | ||
| - | {| style="text-align:center;" | + | {| style="text-align:center; margin: 1em auto 1em auto; padding: 0 10px;" cellpadding="10px" |
|[[File:NTNU_Trondheim_Model_VgbLow.png|thumb|center|450px|Figure 3. Response of vgb promoter to an oxygen level of 10]] | |[[File:NTNU_Trondheim_Model_VgbLow.png|thumb|center|450px|Figure 3. Response of vgb promoter to an oxygen level of 10]] | ||
|[[File:NTNU_Trondheim_Model_VgbHigh.png|thumb|center|450px|Figure 4. Response of vgb promoter to an oxygen level of 250]] | |[[File:NTNU_Trondheim_Model_VgbHigh.png|thumb|center|450px|Figure 4. Response of vgb promoter to an oxygen level of 250]] | ||
| Line 59: | Line 65: | ||
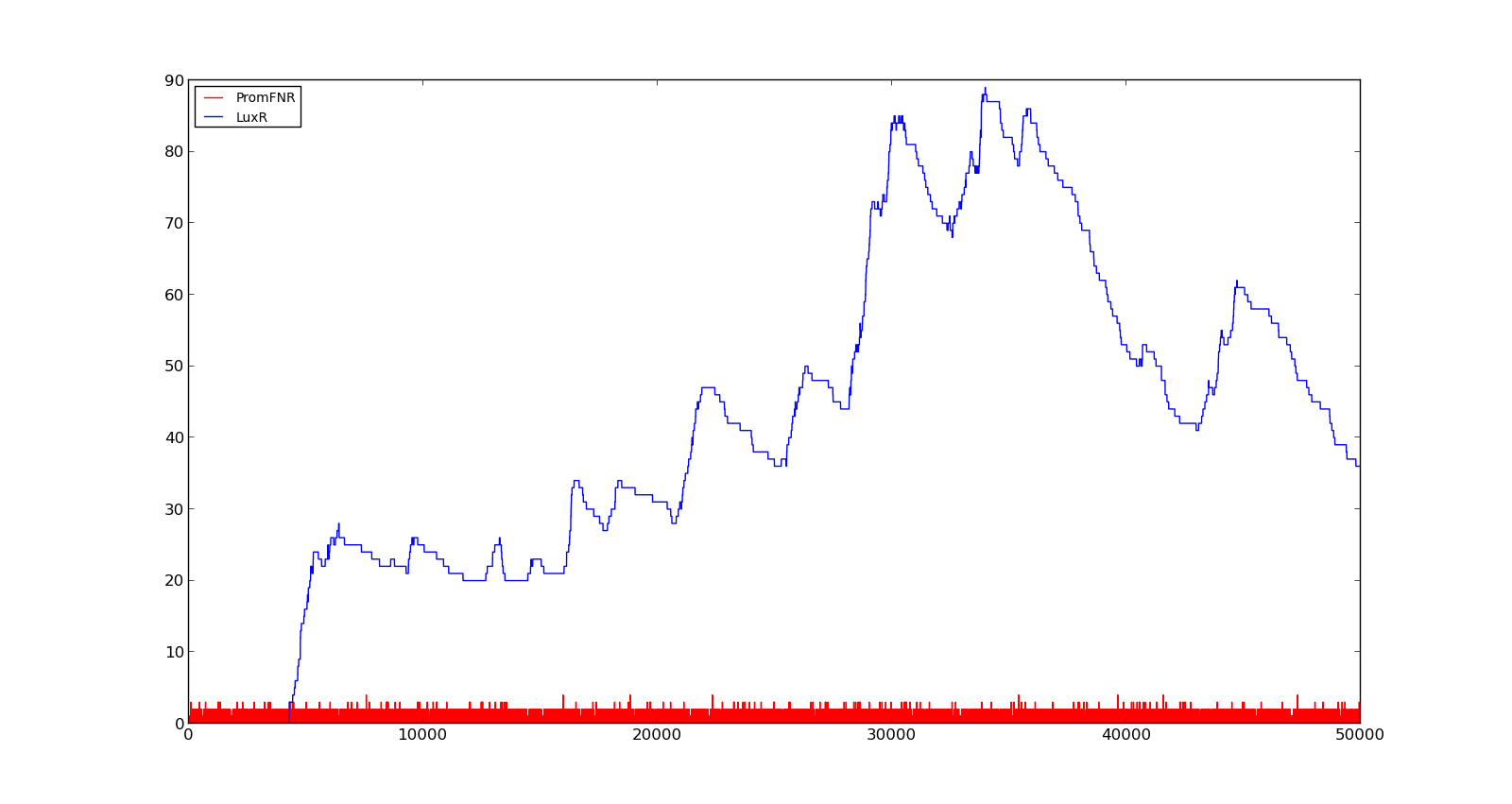
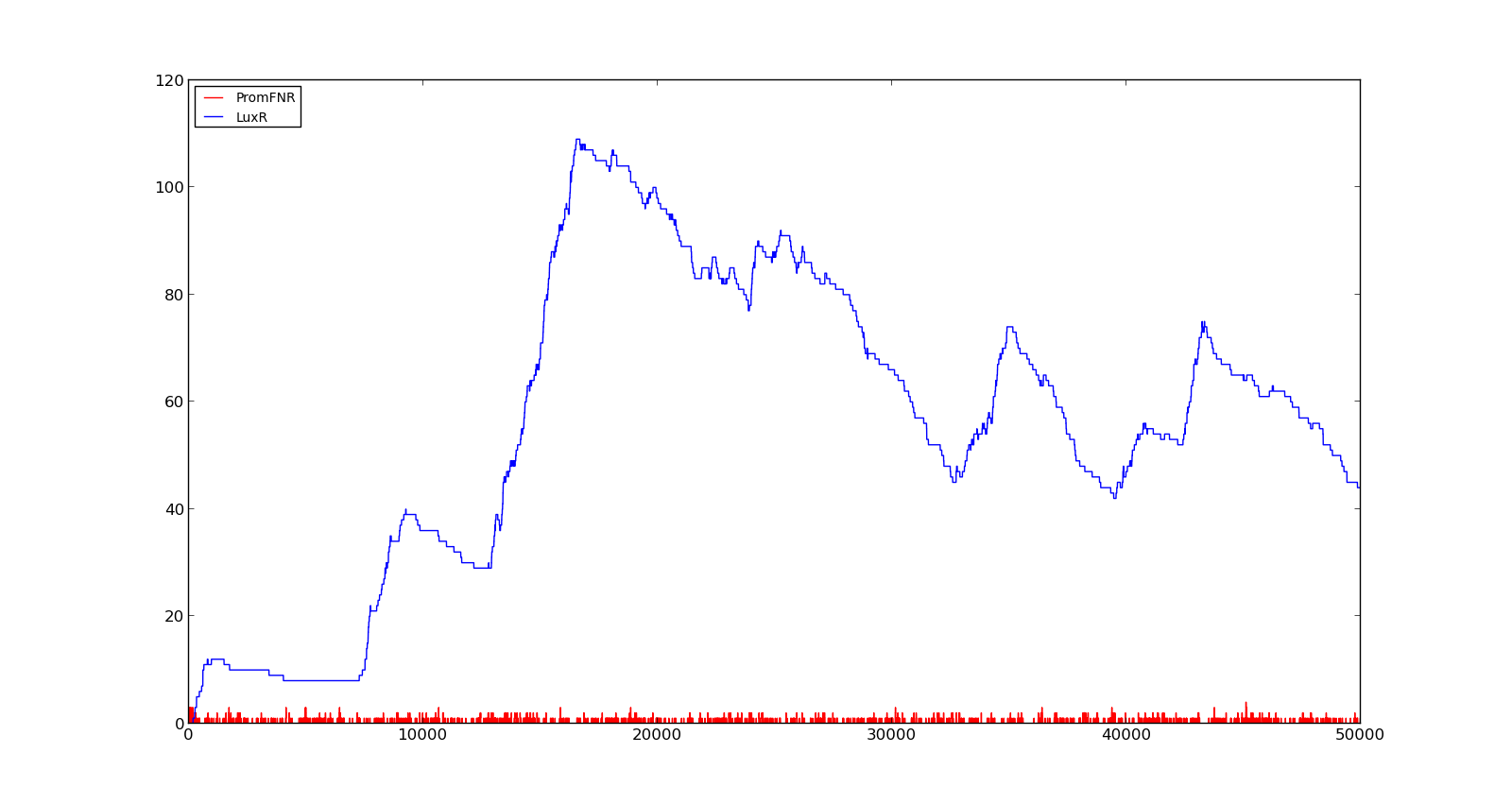
In the cell, two LuxR proteins will form a complex with two HSL molecules. This complex will then bind to a palindromic site and activate the promoter. In the model, the reaction was described as two HSL and two proteins reacting directly and forming a complex that activated the promoter. The results are shown in the figure below. In the figure to the left, a constant production of 0.001 s⁻¹ was assumed for both LuxR and LuxI. This gave an expression of Holin, the compound that cause lysis, see full model, with a maximum about 700. This was quite high, but less than the lethal amount of about a 1000[http://dx.doi.org/10.1146/annurev.micro.54.1.799]. After increasing the expression to 0.01 s⁻¹, the amount increased to about 5500. Again, the amount of activated promoter were quite low, indicating a too high transcription and translation rate. | In the cell, two LuxR proteins will form a complex with two HSL molecules. This complex will then bind to a palindromic site and activate the promoter. In the model, the reaction was described as two HSL and two proteins reacting directly and forming a complex that activated the promoter. The results are shown in the figure below. In the figure to the left, a constant production of 0.001 s⁻¹ was assumed for both LuxR and LuxI. This gave an expression of Holin, the compound that cause lysis, see full model, with a maximum about 700. This was quite high, but less than the lethal amount of about a 1000[http://dx.doi.org/10.1146/annurev.micro.54.1.799]. After increasing the expression to 0.01 s⁻¹, the amount increased to about 5500. Again, the amount of activated promoter were quite low, indicating a too high transcription and translation rate. | ||
| - | {| style="text-align:center;" | + | {| style="text-align:center; margin: 1em auto 1em auto; padding: 0 10px;" cellpadding="10px" |
|[[File:NTNU_Trondheim_Model_LuxBoxLow.png|thumb|center|450px|Figure 6. Response of lux promoter to a LuxI and LuxR production rate of 0.001 s⁻¹]] | |[[File:NTNU_Trondheim_Model_LuxBoxLow.png|thumb|center|450px|Figure 6. Response of lux promoter to a LuxI and LuxR production rate of 0.001 s⁻¹]] | ||
|[[File:NTNU_Trondheim_Model_LuxBoxHigh.png|thumb|center|450px|Figure 7. Response of lux promoter to a LuxI and LuxR production rate of 0.01 s⁻¹]] | |[[File:NTNU_Trondheim_Model_LuxBoxHigh.png|thumb|center|450px|Figure 7. Response of lux promoter to a LuxI and LuxR production rate of 0.01 s⁻¹]] | ||
| Line 71: | Line 77: | ||
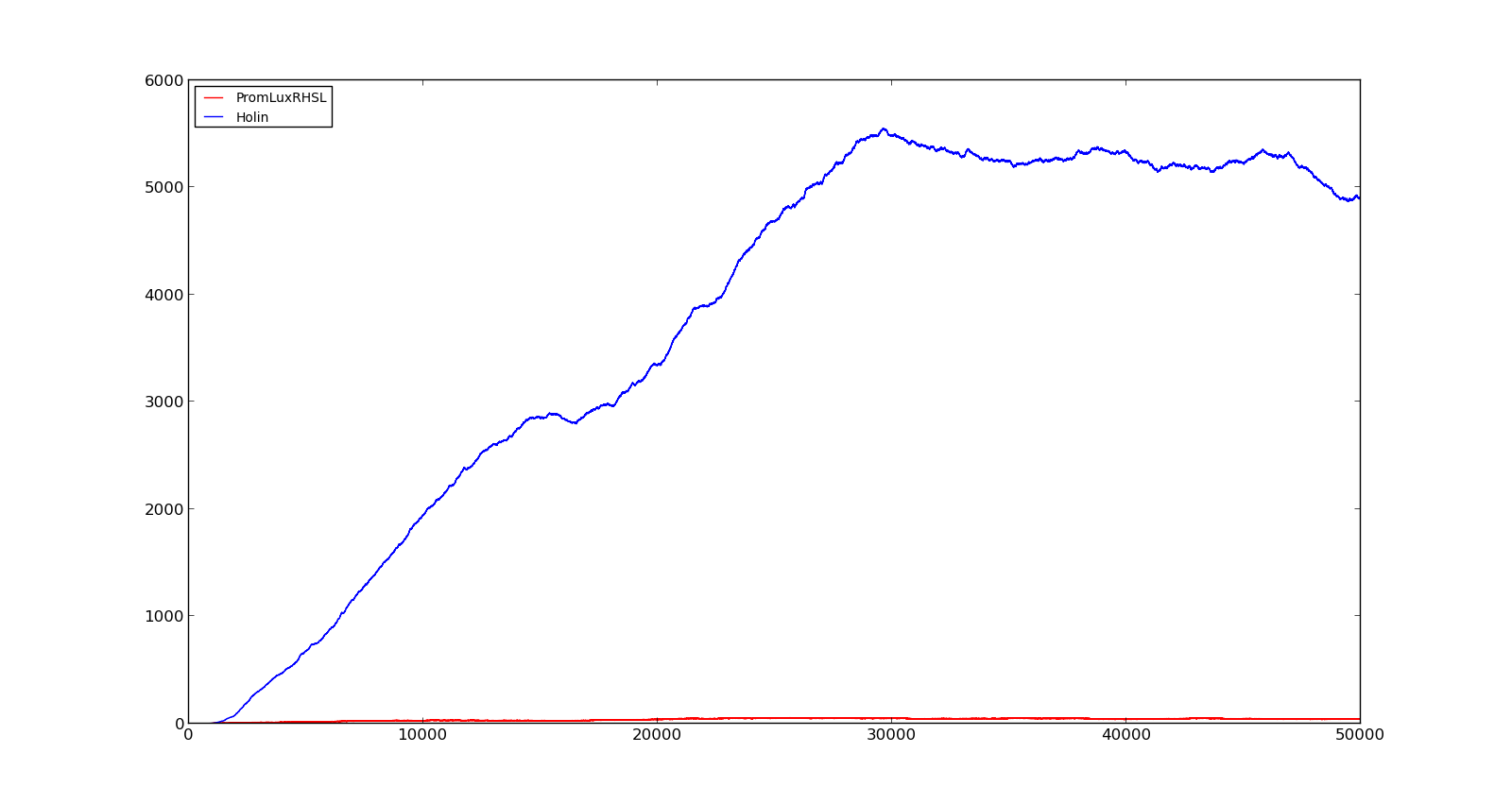
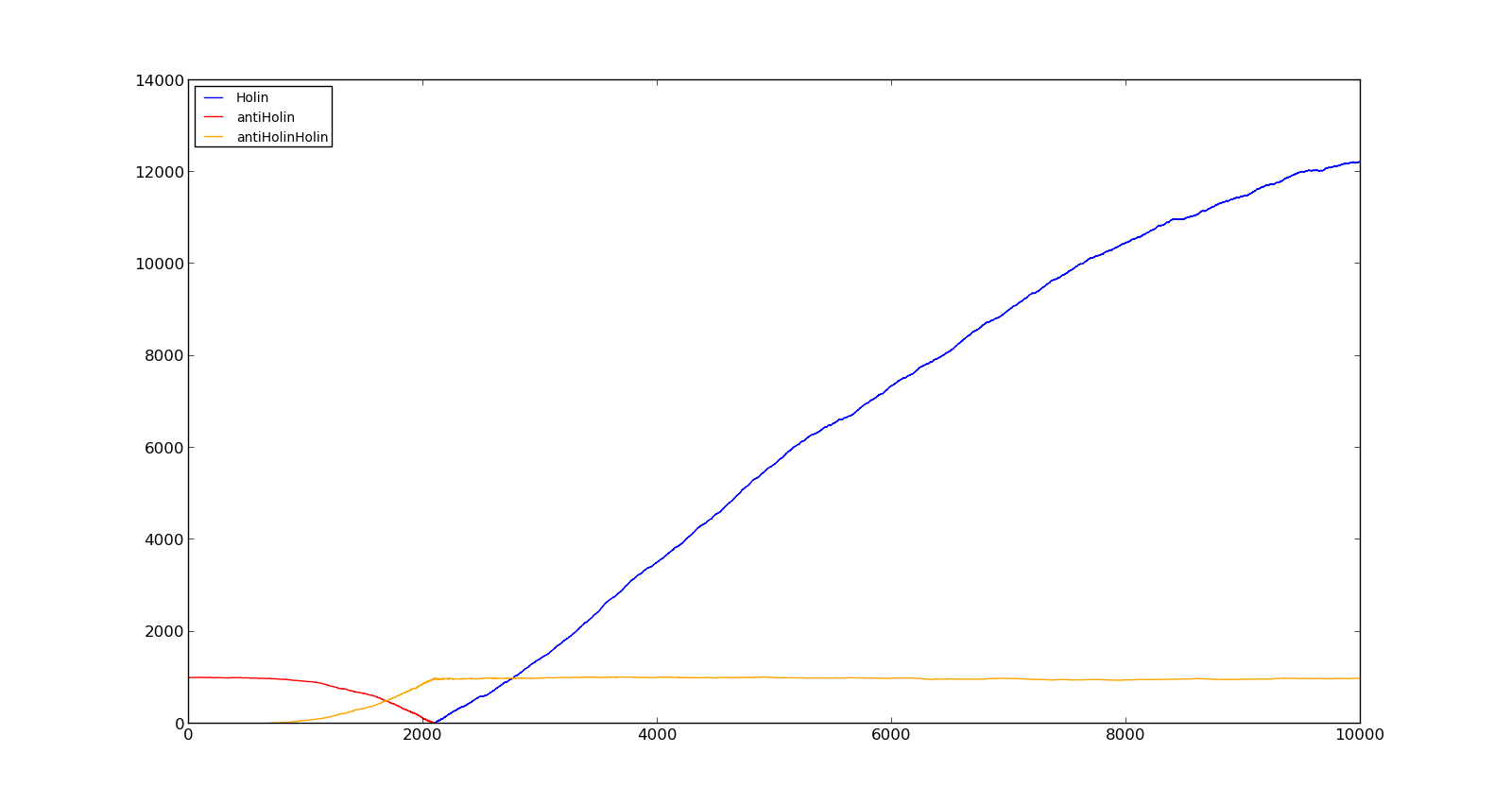
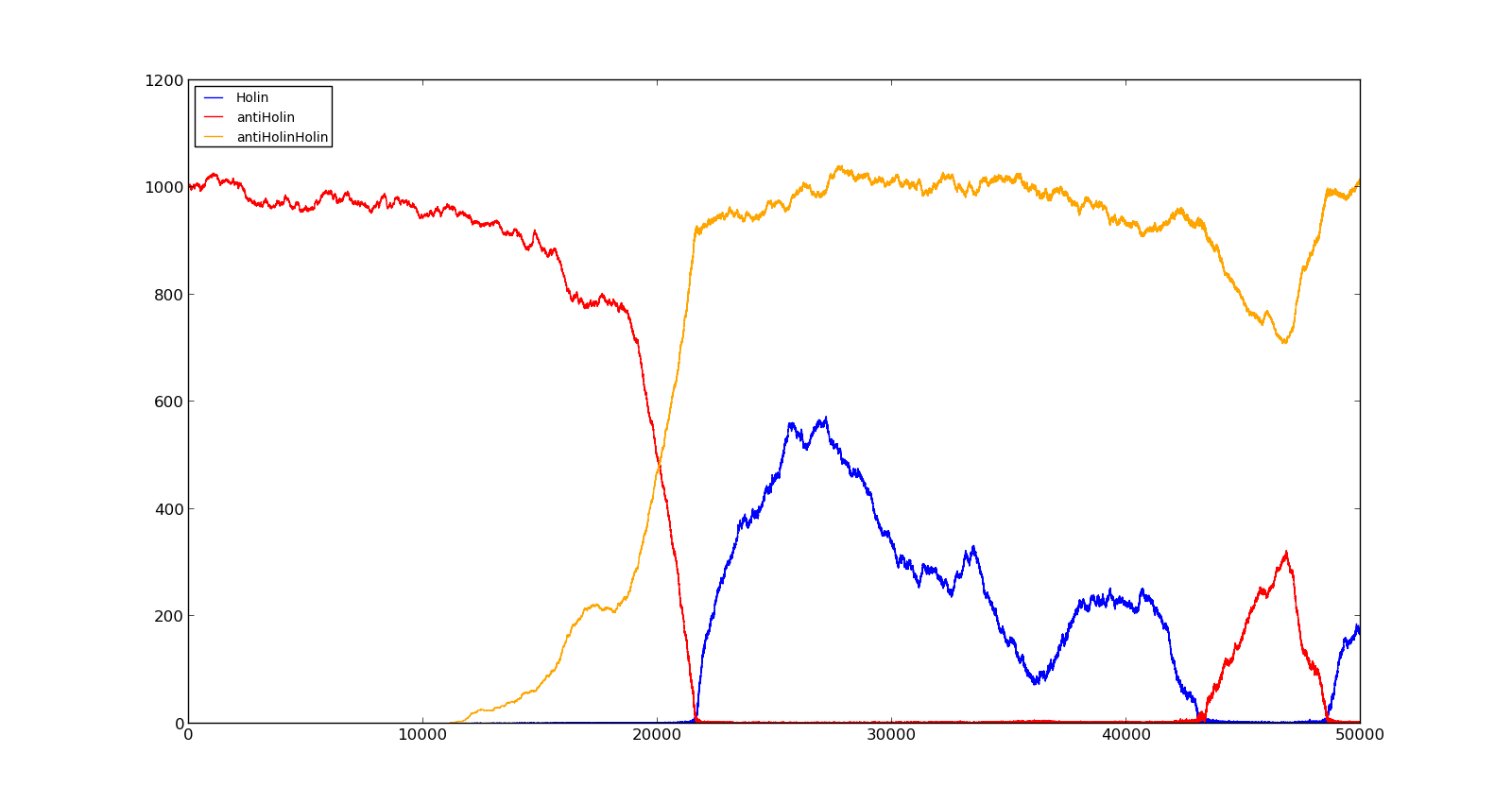
The figures below describe the behaviour of the system under various conditions. Figure 8 shows the system with a relatively low oxygen level of 100 and a high lactate level of 1000. The antiholin will cause a delay in lysis, but holin will reach lethal levels in about an hour. Figure 9 shows the response to oxygen levels of 2000 and lactate levels of 10. In this case, there is still holin production, but most of it is complexed with antiholin and it does not reach lethal levels. | The figures below describe the behaviour of the system under various conditions. Figure 8 shows the system with a relatively low oxygen level of 100 and a high lactate level of 1000. The antiholin will cause a delay in lysis, but holin will reach lethal levels in about an hour. Figure 9 shows the response to oxygen levels of 2000 and lactate levels of 10. In this case, there is still holin production, but most of it is complexed with antiholin and it does not reach lethal levels. | ||
| - | {| style="text-align:center;" | + | {| style="text-align:center; margin: 1em auto 1em auto; padding: 0 10px;" cellpadding="10px" |
|[[File:NTNU_Trondheim_Model_HolinHolinHigh.png|thumb|center|450px|Figure 8. Production rate of holin at oxygen levels of 100 and lactate levels of 1000]] | |[[File:NTNU_Trondheim_Model_HolinHolinHigh.png|thumb|center|450px|Figure 8. Production rate of holin at oxygen levels of 100 and lactate levels of 1000]] | ||
|[[File:NTNU_Trondheim_Model_HolinHolinLow.png|thumb|center|450px|Figure 9. Production rate of holin at oxygen levels of 2000 and lactate levels of 10]] | |[[File:NTNU_Trondheim_Model_HolinHolinLow.png|thumb|center|450px|Figure 9. Production rate of holin at oxygen levels of 2000 and lactate levels of 10]] | ||
| Line 80: | Line 86: | ||
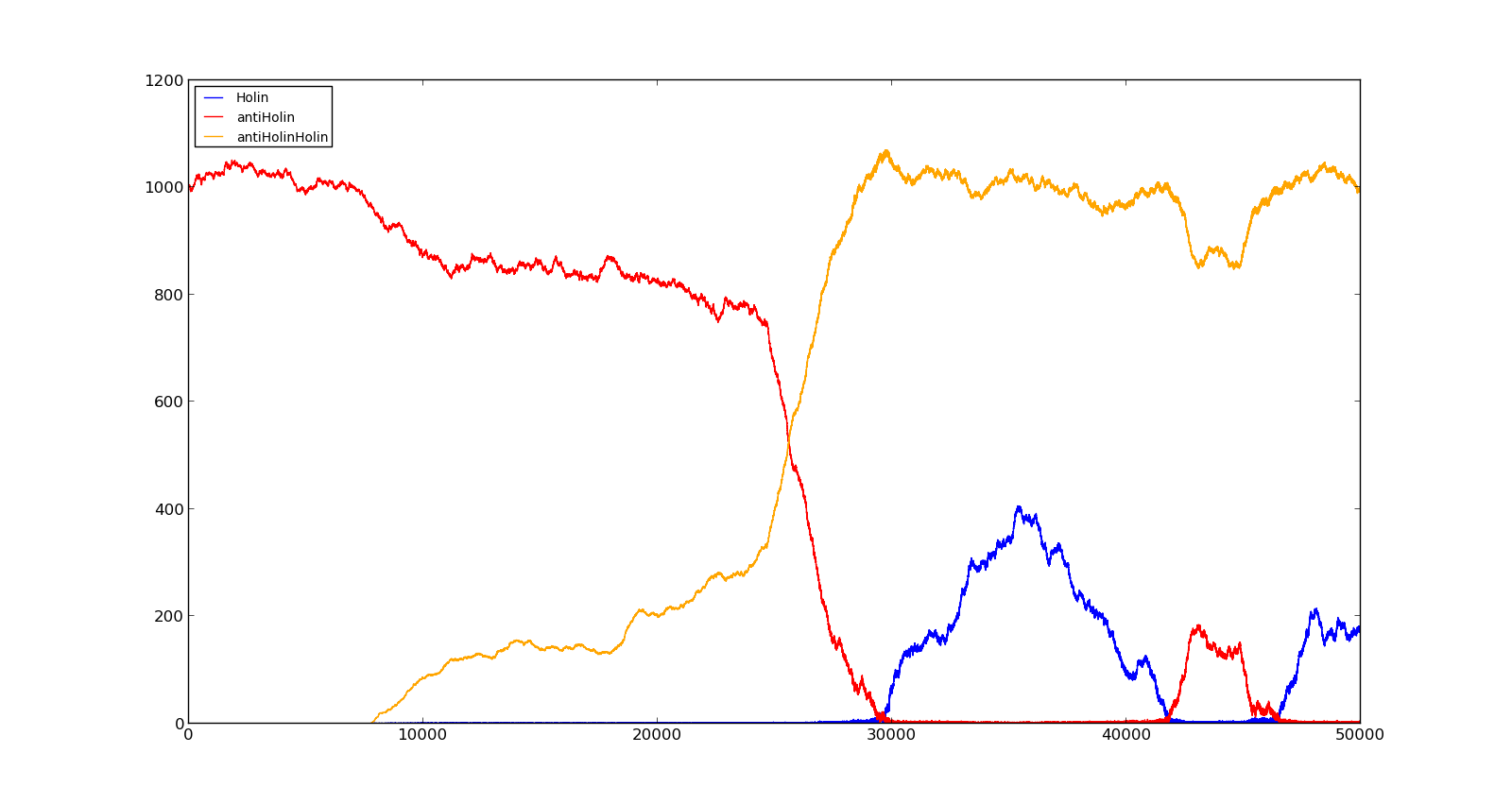
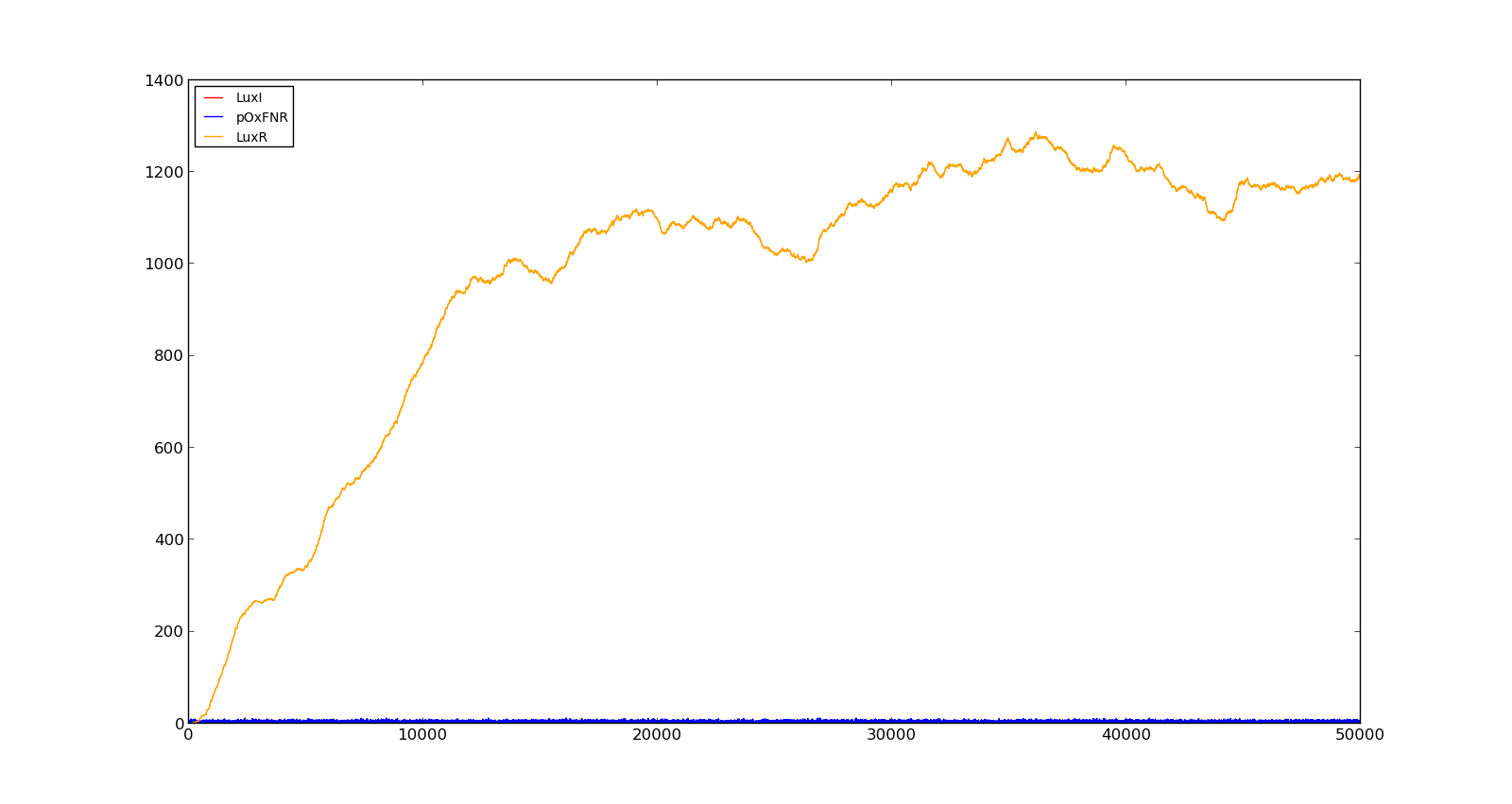
The last plot shows the response to high oxygen levels; 5000, and high lactate levels; 100. this gives a response similiar to the one in Figure 9 were holin is produced, but forms a complex with antiholin and does not reach large enough quantities to cause lysis. | The last plot shows the response to high oxygen levels; 5000, and high lactate levels; 100. this gives a response similiar to the one in Figure 9 were holin is produced, but forms a complex with antiholin and does not reach large enough quantities to cause lysis. | ||
| - | {| style="text-align:center;" | + | {| style="text-align:center; margin: 1em auto 1em auto; padding: 0 10px;" cellpadding="10px" |
|[[File:NTNU_Trondheim_Model_HighOxNoLact.png|thumb|center|450px|Figure 10. Production rate of LuxR and LuxI at oxygen levels of 250 and no lactate]] | |[[File:NTNU_Trondheim_Model_HighOxNoLact.png|thumb|center|450px|Figure 10. Production rate of LuxR and LuxI at oxygen levels of 250 and no lactate]] | ||
|[[File:NTNU_Trondheim_Model_LactVHighOx.png|thumb|center|450px|Figure 11. Production rate of holin at oxygen levels of 5000 and lactate levels of 100]] | |[[File:NTNU_Trondheim_Model_LactVHighOx.png|thumb|center|450px|Figure 11. Production rate of holin at oxygen levels of 5000 and lactate levels of 100]] | ||
|} | |} | ||
| + | |||
| + | ==Modelling for RHIT== | ||
| + | |||
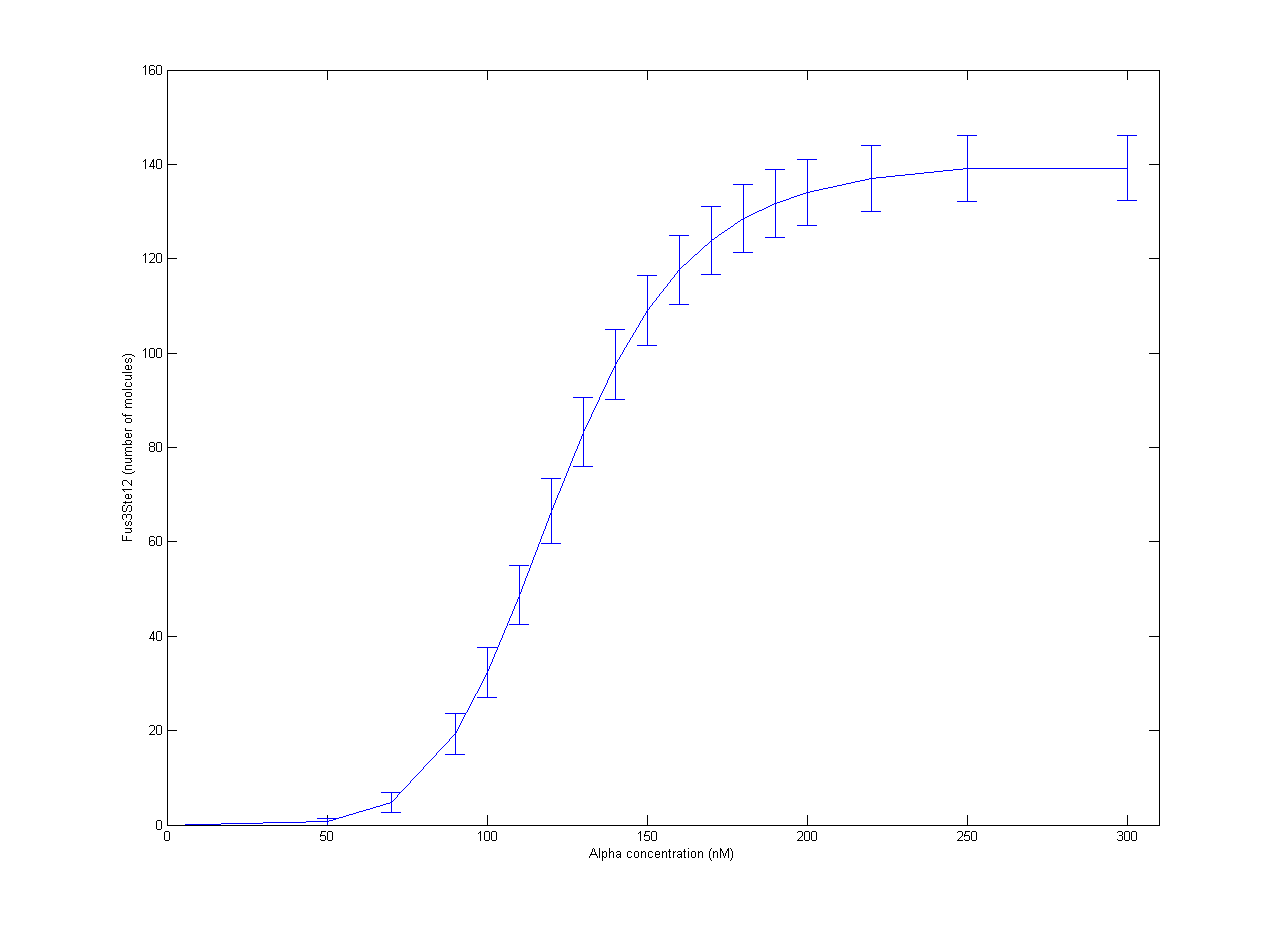
| + | Our part of the [https://2012.igem.org/Team:NTNU_Trondheim/Collaboration collaboration] with RHiT was helping them with stochastic modelling of the trigger system for mating in yeast. The full mechanism has been quite well studied, but it is very complicated[http://dx.doi.org/10.1002/yea.1122]. In RHiTs [[Team:RHIT/Modeling|model]], the final steps of the mechanism is activation of the Ste12 protein by the Fus3 enzyme. To simplify the model, production of Fus3 in the model was described by a sigmoid curve found in experiments[http://dx.doi.org/10.1038/nature08946] with respect to the concentration of α-pheromone. Inactive Ste12 was quickly activated by the presence of Fus3, so the outcome of active Ste12 followed a similar sigmoid curve, giving the expected switch behaviour. The resulting plot is shown in Figure 1. Each point is the average of 100 trajectories with the error bars indicating one standard deviation. | ||
| + | |||
| + | [[File:NTNU_Trondheim_YeastAlpha.png|thumb|center|450px|Figure 1. Amount of activated Ste12 at steady state as a response to α-pheromone concentrations. Error bars show one standard deviation.]] | ||
| + | |||
| + | The equations used in the model are given in the table below. The parameters are taken from[http://dx.doi.org/10.1002/yea.1122 [1<nowiki>]</nowiki>] and are modelled using mass action solvers, except Fus3 → Fus3PP, which use a sigmoid function. Timesteps in the model are minutes. | ||
| + | |||
| + | {|class="table table-bordered table-hover" style="margin: 1em auto 1em auto; width: auto;" | ||
| + | |- | ||
| + | !Reaction | ||
| + | !Propensity | ||
| + | !Comment | ||
| + | |- | ||
| + | |Fus3 → Fus3PP | ||
| + | |* | ||
| + | |Activation of Fus3 | ||
| + | |- | ||
| + | |Fus3PP → Fus3 | ||
| + | |150 | ||
| + | |Deactivation of Fus3 | ||
| + | |- | ||
| + | |Fus3PP + Ste12 → Fus3Ste12 | ||
| + | |18 | ||
| + | |Activation of Ste12 through complexation with Fus3 | ||
| + | |- | ||
| + | |Fus3Ste12 → Fus3PP + Ste12 | ||
| + | |10 | ||
| + | |Deactivation of Ste12 by release of Fus3 | ||
| + | |- | ||
| + | |Bar1 + Fus3Ste12 → aBar1 + Fus3Ste12 | ||
| + | |0.1 | ||
| + | |Activation of Bar1 enzyme | ||
| + | |- | ||
| + | |aBar1 → Bar1 | ||
| + | |0.1 | ||
| + | |Deactivation of Bar1 | ||
| + | |- | ||
| + | |aBar1 → ø | ||
| + | |0.01 | ||
| + | |Export of active Bar1 | ||
| + | |} | ||
| + | ''* The function for Fus3 activation is given by'' 200*α⁶/(α⁶ + 150⁶) ''where α is the concentration of α-phermone in nM'' | ||
| + | |||
| + | The initial amounts are given in the table below. | ||
| + | |||
| + | {|class="table table-bordered table-hover" style="margin: 1em auto 1em auto; width: auto;" | ||
| + | |- | ||
| + | !Species | ||
| + | !Amount | ||
| + | |- | ||
| + | |Fus3 | ||
| + | |200 | ||
| + | |- | ||
| + | |Fus3PP | ||
| + | |0 | ||
| + | |- | ||
| + | |Ste12 | ||
| + | |200 | ||
| + | |- | ||
| + | |Fus3Ste12 | ||
| + | |0 | ||
| + | |- | ||
| + | |Bar1 | ||
| + | |200 | ||
| + | |- | ||
| + | |aBar1 | ||
| + | |0 | ||
| + | |} | ||
| + | |||
| + | A .zip file of the model can be downloaded [[Media:NTNU_Trondheim_Yeast.zip|here]]. The original file is in .xml format and can be opened with the Cain software[http://cain.sourceforge.net]. | ||
| + | |||
| + | {{:Team:NTNU_Trondheim/Templates/Sponsors}} | ||
| + | <html> | ||
| + | </div></div></html> | ||
| + | {{:Team:NTNU_Trondheim/Templates/Footer}} | ||
Latest revision as of 23:26, 26 September 2012
 "
"