Team:Evry/GB
From 2012.igem.org
| Line 101: | Line 101: | ||
<p>Because it requires a very small amount of DNA, the plasmid source can be taken directely from a DNA distribution suck as the partsregistry distribution, without requiering the need of a DNA preparation before. Once the plasmid are resuspended, the experimentalist mix them together in a mix containing the BsaI enzyme and T4 ligase and put them in the thermocycler for 5 hours. In the end, the product can be directely transformed and plated. A first round of screening sor the good polymerization lenght can be made using colony PCR with the standard VF2 and VR primers. If required, a second step of screening can be made after minipreping using BbsI. Given the very small rate of mutation, only two clones with the good digestion or colony PCR signature can be sent for sequencing; they will contain the correct construct with a very high probability.</p> | <p>Because it requires a very small amount of DNA, the plasmid source can be taken directely from a DNA distribution suck as the partsregistry distribution, without requiering the need of a DNA preparation before. Once the plasmid are resuspended, the experimentalist mix them together in a mix containing the BsaI enzyme and T4 ligase and put them in the thermocycler for 5 hours. In the end, the product can be directely transformed and plated. A first round of screening sor the good polymerization lenght can be made using colony PCR with the standard VF2 and VR primers. If required, a second step of screening can be made after minipreping using BbsI. Given the very small rate of mutation, only two clones with the good digestion or colony PCR signature can be sent for sequencing; they will contain the correct construct with a very high probability.</p> | ||
| - | <p>This technique however requires to screen an important number of clones because we don't have a total control of what is assembling inside the cassette, because of the freedom we left for the RBS-Protein entities to polymerize. We will show in the next paragraph how we can gain a relative control over it. To reduce the costs of screening, we encourage the use of colony PCR oven mini-preping and digestion when possible. | + | <p>This technique however requires to screen an important number of clones because we don't have a total control of what is assembling inside the cassette, because of the freedom we left for the RBS-Protein entities to polymerize. We will show in the next paragraph how we can gain a relative control over it. To reduce the costs of screening, we encourage the use of colony PCR oven mini-preping and digestion when possible.</p> |
<h2>Control of the polymerization</h2> | <h2>Control of the polymerization</h2> | ||
| + | |||
| + | <p>The only difference with a standard GoldenBrick protocol is the capacity for the RBS-protein cassette to polymerize. This polymerization capacity makes the power of the technique because it enable to make polysistronic mRNAs no matter what gene is inserted. However, a control have to be achieved oved it in order to control when to create polysistrons and when we want a single gene to be inserted.</p> | ||
| + | |||
| + | <p>In order to acheive such a control, several parameters in the protocol will move the balance from mono-systrons to poly-sistrons. The firt and the most obvious one is the stoechimoetry of the different elements. If the promoter and terminators are in large excess compared to the RBSs and the gene, the balance will be in favor of mono-sistrons. On the contrary, if the amount or RBSs and gene in dominant, the cassette will tends to contain two or more genes inserted inside.</p> | ||
| + | |||
| + | <p>On the other hand, the number of cycle and the ligation time will also influence the polymerization. Poly-sistrons are not likely to appear when the number of cycle is small and the ligation time short, but they will become more and more important as we increase these parameters.</p> | ||
| + | |||
| + | <p>Most of the work on GoldenBrick by our team is now focused in optimizing the protocol. We will release soon a set of standard protocols for mono and polysistrons optimized to reduce the polydispersity of the polymerization lenght.</p> | ||
<h2>Non standard assembly and "split-construct" vectors</h2> | <h2>Non standard assembly and "split-construct" vectors</h2> | ||
| + | |||
| + | <p>If you are willing ot create a non standard assembly or to control very precisely RBS-protein pairing over a polysistrons, you might like to create the construct in two times and assemble them together in a second step. This is why we have created the "split-construct" vectors. The split-contruct vectors are like the goldenbrick vectors except that they contain the OH2-OH5 or the OH1-OH4 overhangs instead of the OH1-OH5 overhangs as the GoldenBrick plasmid.</p> | ||
| + | |||
| + | <p>These vectors enable the assembly of shorter casette containing only a promoter, and a serie of RBS-protein for the "split-construct" 1 vector, or RBS-protein terminator for the "split-construct" 2 vector. This way, if a non standard biobrick assembly have to be cassied in wetween the two pieces or if a long polysistron have to be created, they can be assembled using the standard EcoRI, XbaI, SpeI, PstI cloning, but several cloning step would have been saved by using GoldenBricks compared to a standard assembly.</p> | ||
| + | |||
| + | <h2>GoldenBrick make standard part DNA shuffeling possible</p> | ||
| + | |||
| + | <p>Aside from the dramatic shortening of the cloning time, the decrease of the costs and the easiness of GoldenBricks over Biobricks, one of the most noticeable improvement is undoubtely the possiblity to make DNA shuffling using a standard format. Lets take the example of a complex system such as a toogle-switch, in which you would like to optimize the expression of the protein repression of its two component to place the system in its working domain.</p> | ||
| + | |||
| + | <p>Using the Biobrick format, you will probably have to make tens of different constructs with different RBS to kind a condition in which the toogle-switch will work. Using GoldenBricks, you can make all of them in a single tube, in a single step. Moreover, you may eventually find a way to screen them directely after the cloning avoiding the need of purifying and sequence all the clones that couldn't work. The figure XX shows in theory how one can proceed to such experiment design.</p> | ||
| + | |||
| + | <p>Many other use of DNA shuffling can be find in the litterature expecially for gene expression optimization and screening for working enzymes. We expect that DNA shuffling over standard parts is going to find many applications in the future of iGEM projects.</p> | ||
<h2>GoldenBrick is still compatible with Biobrick format</h2> | <h2>GoldenBrick is still compatible with Biobrick format</h2> | ||
| - | <h2>Classical GoldenGate can be | + | <p>If the situation requires the part to be assembled with the standard RFC 10 format, the new GoldenBrick parts can be assembled the very same way than BioBricks. The only limitation is that BioBrick assembly of GoldenBrick parts leave a scar containing the BsaI restriction site inside, and the produced parts cannot be reassembled with other parts using GoldenGate. No simple way of fixing this issue has been found.</p> |
| + | |||
| + | <h2>GoldenBrick also assemble PCR products and shorten the assembly time</h2> | ||
| + | |||
| + | |||
| + | |||
| + | <h2>Classical GoldenGate can be mixed GoldenBrick assembly to create fusion proteins</h2> | ||
| + | |||
| + | <h2>Finally, GoldenBricks works with eukaryotes chassis as well as with prokaryotes</h2> | ||
<h1>Results</h1> | <h1>Results</h1> | ||
Revision as of 20:27, 21 September 2012
The GoldenBricks: A new and fast cloning technique for iGEM

Introduction
Context
Cloning is probably the most tedious and less rewarding part of the work when engineering living entities in synthetic biology. With the devlopment of the restriction enzymes and efficient DNA ligases, assembling small and large pieces of DNA has become a reality, but as everyone was using his own enzymes and assembly method, making large constructs was always a real challenge in itself. The first indisputable advance in the domain came with the invention the first standardized DNA parts: BioBricks. The standard BioBrick format (BBF RFC 10) made possible to clone together all kind of genetic parts (called bricks) in a standard way using only 4 different enzymes, and made possible the creation of a huge DNA database containing several thousands of described and characterized biological parts: the PartsRegistry.
If biobricks have opened new perspectives for engineering new organisms and has proven to its efficiency over years, this technique can only join two parts at the time. Moreover, it is still very difficult to automatize biobrick's assembly because of the important number of DNA purification step required. iGEMers today's spent most of their time assembling their genetic parts together, leaving little characterization and tests.
Several cloning techniques enabling the assembly of multiple parts at the time has been invented since the rise of the Biobrick format. The most popular one is probably the one known as the Gibson method [] that can be use to assemble up to 4 fragments at the time on a regular basis. The Golden Gate technique [] (>20 fragments at the time) and its new evolution, the MoClo [] (47 fragments in two times reported) are leading the way of another kind of cloning technique based on type II restriction enzymes. More efficient than the Gibson cloning, they also have the huge avantage not to imply DNA modifications, which reduces severely the probability of having a mutation at the ligation scar. If theses new techniques dramatically speed-up the assembly of DNA pieces, to our knowledge, no reported work has been carried toward standardizing these methodologies.
Because of the overlapping region's size required by Gibson, this technique is impossible to standardize if we want to keep a close distance between the parts as required. Type II restriction enzyme cloning is more adapted because the engineerable overhangs are only 4 nucleotides long and preserve the distances. So far, when people were doing goldengate, new overhangs are engineered each times, and therefore, a new library has to be constructed each time.
From Golden Gate and BioBricks to GoldenBricks
The biobricks are a collection of parts that can be assembled one by one in a generic way. Golden Gate is a technique that enable the assembly of several tens of parts simultaneously. Taking inpiration from both technique, the 2012 Evry iGEM Team have invented and developped a new methodology called GoldenBricks, merging the power of the two techniques while keeping the compatibility toward the old RFC 10 BioBrick format and a compatibility with the GoldenGate assembly technique.
This technique enable one-shot cassette construction using DNA parts coming either from a plasmid distribution or from PCR product, engaging up to seven different parts. Moreover, goldenbricks works for both eukaryotes and procaryotes DNA construction with the very same protocol. If a non classical assembly is required (as for testing the strenght of a terminator) a new "split construction" method based on standard plasmids make possible to assemble parts in a non classical way.
Perspectives
Such technique offers great perspectives for the future of iGEM and of the partsregistry in general. First, it would make possible the fast and simple assembly of complete cassettes using less expensive equipment, toxic chemical and sequencing runs than the BioBrick assembly. It would make fast and efficient cloning accessible for both researchers as well as for less experimented users, such as high school iGEMers or biohackers because of the simplicity of the process. Second, the creation of large expression cassettes using this method goes cheaper and faster than synthesizing the construct at the present day, which would guaranty the interest for a DNA database compared to de-novo synthesis. And last, this method is a lot more easily automatable contrary to RFC 10 biobricks.
Theory
Requirements for the development of the new standard
As we said previously we tried in this work to stay as close as possible to biobrick format, to keep the parts compatible with the RFC 10 standard assembly, while opening new perspectives to increase the speed and the easiness of cloning. These constraints impose several requirements for this new standard.
First, the new assembly method should:
- Keep the compatibility with the standard RFC 10
- Be compatible with a database approach
- Minimize the number of different prefix and suffix
- Use the standard registry plasmid pSB1C3 and the J04450 cloning negative control
To improve the cloning speed, we would also like to:
- Enable one step golden-gate cassette cloning
- Allow to check the construct with a single digestion
- Improve the compatibility with Gibson
- Provide a solution for non standard assembly
As we will demonstrate, the GoldenBrick format meet all these requirement. We are now going to explain you the principles that led the design of this format before we analyze the sequences of the GoldenBrick prefixes and suffixes.
Analysis of a classical synthetic biology constructions
A traditional construct in synthetic biology is made of the repetition of the following elements:

Fig 1: Schematic of the different elements assembly in a standard synthetic biology device.
At the moment, GoldenBrick only allow the assembly of a cassette at the time. In order to create the full system, the assembly of the different cassettes together have to be conducted afterwards with the biobrick assembly of with Gibson assembly, that is very efficient to assemble long fragments. Focusing closer on a single cassette, we can notice that we have 4 different kind of junctions:

Fig 2:In a single cassette, we can notice 4 different kinds of junction. If we want the polymerization to be possible, the prefix of the RBS have to match with the suffix of the gene.
As we can notice on this picture, there is 4 different kind of junction in this kind of device. In Golden Gate, the junctions have the form of a DNA overhang that we can freely engineer. Therefore, we should engineer 4 different overhangs. The overhang overhang is dedicated to the ligation of the plasmid suffix with the promoter prefix (OH1). Similarely, the 5th one is dedicated to ligate the terminator suffix with the backbone prefix (OH5). In a bacterial device, the scar between the RBS and the gene (OH3) has to be very well controlled because the distance between the two elements is critical for correct protein expression. This scar will remain the same than in the biobrick assembly, but it will be generated by a type II enzyme as for the others overhangs. Finally, the overhang that link the RBS prefix to the promoter suffix (OH2) and the protein suffix to the terminator prefix (OH4) have to be compatible to make possible the repetition of several {RBS-Protein} units in a single cassette (OH2=OH4). We will see later how to control this repetition.
To conclude, the necessity for 4 different overhangs implies to create 4 different prefixes and 4 different suffixes depending on the function of the DNA elements.
In addition, we would like the scar generated by the assembly of all the different elements to be digestible by a single enzyme. This would enable to control whether all the elements we intended to insert in the construct has been assembled before sequencing the casette. As we will see later this element will be critical for the selection of the clone that have inserted all the genes we want. This is why the overhangs 1, 2, 4 and 5 has been engineered in the middle of a BbsI site. When two pieces ligates, they recreate a BbsI restriction site, and we can check the correct insertion of a given gene in the construct by simply digesting with BbsI and checking for the size on a gel.
One last condition to fulfil to get a RFC 10 compatible brick after GoldenBrick cloning is to leave no illegal restriction site inside the brick after the assembly of the cassette. However, we should keep the as similar as possible the biobrick extensions around each elements leaving the 4 usuals enzyme restriction sites, so that the brick can be still assembled using biobrick method. The only drawback we didn't managed to overcome is that if several GoldenBrick parts are assembled with RFC 10 standard, they cannot be assembled together using GoldenGate afterwards, because the biobrick assembly will leave illegal BsaI site inside the brick.
The proposed new set of Golden Bricks extension
Having all these design principles in mind and meeting the requirements we have proposed, we designed the new GoldenBrick prefix and suffix with the following sequences:

Figure 3:Sequence and design of the different prefixes and suffixes used in the GoldenGate cloning method. The presence of the EcoRI, XbaI, SpeI and PstI keep the compatibility with Biobrick cloning while the BsaI site enable single shot GoldenGate assembly. The BbsI sites serve as a single digestion control.
This design fulfil all the requirements enumerated before and ensure that the different elements will be assembled in the correct order. We also generate a scar that can be digested afterwards with the Bbsi enzyme, enabling to check of the correct insertion of the different elements with the help of a single digestion. This also enable the polymerization, which we will discuss in the next paragraph.
Assembly procedure
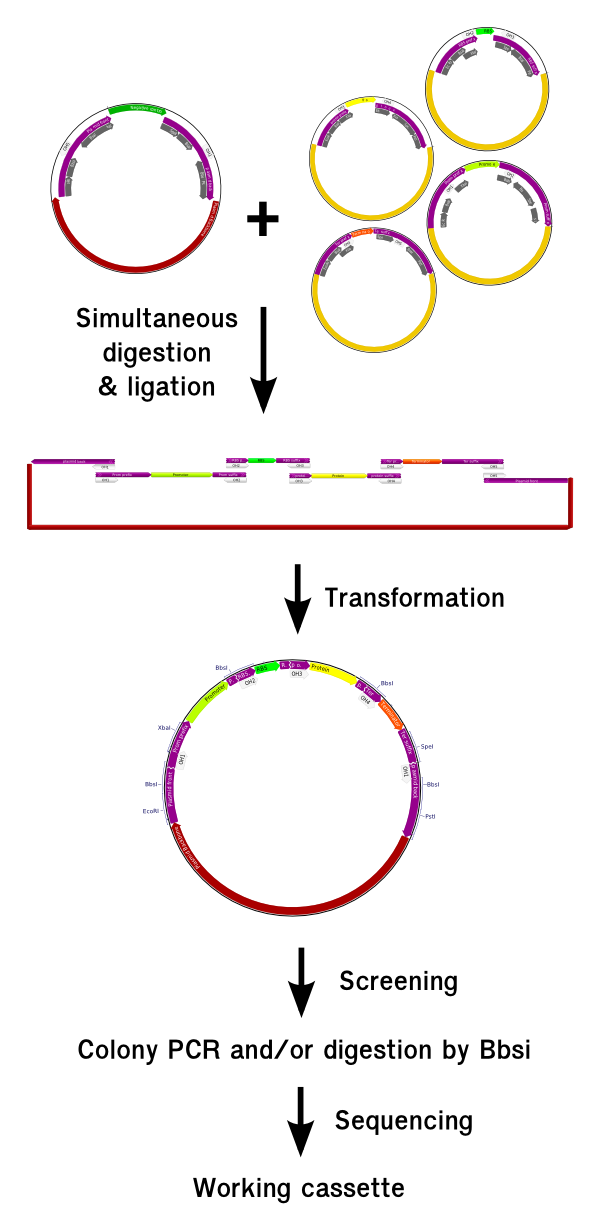
Assembling parts using the GoldenBrick method is a lot easier than for BioBricks and also 5 to 10 times faster. We are now going to explain the procedure from the theoritical point of view. For the complete protocol, you should refers to the material and method section.
Because it requires a very small amount of DNA, the plasmid source can be taken directely from a DNA distribution suck as the partsregistry distribution, without requiering the need of a DNA preparation before. Once the plasmid are resuspended, the experimentalist mix them together in a mix containing the BsaI enzyme and T4 ligase and put them in the thermocycler for 5 hours. In the end, the product can be directely transformed and plated. A first round of screening sor the good polymerization lenght can be made using colony PCR with the standard VF2 and VR primers. If required, a second step of screening can be made after minipreping using BbsI. Given the very small rate of mutation, only two clones with the good digestion or colony PCR signature can be sent for sequencing; they will contain the correct construct with a very high probability.
This technique however requires to screen an important number of clones because we don't have a total control of what is assembling inside the cassette, because of the freedom we left for the RBS-Protein entities to polymerize. We will show in the next paragraph how we can gain a relative control over it. To reduce the costs of screening, we encourage the use of colony PCR oven mini-preping and digestion when possible.
Control of the polymerization
The only difference with a standard GoldenBrick protocol is the capacity for the RBS-protein cassette to polymerize. This polymerization capacity makes the power of the technique because it enable to make polysistronic mRNAs no matter what gene is inserted. However, a control have to be achieved oved it in order to control when to create polysistrons and when we want a single gene to be inserted.
In order to acheive such a control, several parameters in the protocol will move the balance from mono-systrons to poly-sistrons. The firt and the most obvious one is the stoechimoetry of the different elements. If the promoter and terminators are in large excess compared to the RBSs and the gene, the balance will be in favor of mono-sistrons. On the contrary, if the amount or RBSs and gene in dominant, the cassette will tends to contain two or more genes inserted inside.
On the other hand, the number of cycle and the ligation time will also influence the polymerization. Poly-sistrons are not likely to appear when the number of cycle is small and the ligation time short, but they will become more and more important as we increase these parameters.
Most of the work on GoldenBrick by our team is now focused in optimizing the protocol. We will release soon a set of standard protocols for mono and polysistrons optimized to reduce the polydispersity of the polymerization lenght.
Non standard assembly and "split-construct" vectors
If you are willing ot create a non standard assembly or to control very precisely RBS-protein pairing over a polysistrons, you might like to create the construct in two times and assemble them together in a second step. This is why we have created the "split-construct" vectors. The split-contruct vectors are like the goldenbrick vectors except that they contain the OH2-OH5 or the OH1-OH4 overhangs instead of the OH1-OH5 overhangs as the GoldenBrick plasmid.
These vectors enable the assembly of shorter casette containing only a promoter, and a serie of RBS-protein for the "split-construct" 1 vector, or RBS-protein terminator for the "split-construct" 2 vector. This way, if a non standard biobrick assembly have to be cassied in wetween the two pieces or if a long polysistron have to be created, they can be assembled using the standard EcoRI, XbaI, SpeI, PstI cloning, but several cloning step would have been saved by using GoldenBricks compared to a standard assembly.
GoldenBrick make standard part DNA shuffeling possible
Aside from the dramatic shortening of the cloning time, the decrease of the costs and the easiness of GoldenBricks over Biobricks, one of the most noticeable improvement is undoubtely the possiblity to make DNA shuffling using a standard format. Lets take the example of a complex system such as a toogle-switch, in which you would like to optimize the expression of the protein repression of its two component to place the system in its working domain.
Using the Biobrick format, you will probably have to make tens of different constructs with different RBS to kind a condition in which the toogle-switch will work. Using GoldenBricks, you can make all of them in a single tube, in a single step. Moreover, you may eventually find a way to screen them directely after the cloning avoiding the need of purifying and sequence all the clones that couldn't work. The figure XX shows in theory how one can proceed to such experiment design.
Many other use of DNA shuffling can be find in the litterature expecially for gene expression optimization and screening for working enzymes. We expect that DNA shuffling over standard parts is going to find many applications in the future of iGEM projects.
GoldenBrick is still compatible with Biobrick format
If the situation requires the part to be assembled with the standard RFC 10 format, the new GoldenBrick parts can be assembled the very same way than BioBricks. The only limitation is that BioBrick assembly of GoldenBrick parts leave a scar containing the BsaI restriction site inside, and the produced parts cannot be reassembled with other parts using GoldenGate. No simple way of fixing this issue has been found.
GoldenBrick also assemble PCR products and shorten the assembly time
Classical GoldenGate can be mixed GoldenBrick assembly to create fusion proteins
Finally, GoldenBricks works with eukaryotes chassis as well as with prokaryotes
Results
Creation of a simple cassette
Polymerization statistics
Non classical constructions/h2>
Working with regular GoldenGate using GoldenBricks vectors
Material and methods
Construction of the library
Assembly protocol
Perspectives in implementing GoldenBricks in the PartsRegistry
Conclusion
References:
- An introduction to agent-based modeling: Modeling natural, social and engineered complex systems with NetLogo, Wilensky, U., & Rand, W. (in press), Cambridge, MA: MIT Press
References:
- An introduction to agent-based modeling: Modeling natural, social and engineered complex systems with NetLogo, Wilensky, U., & Rand, W. (in press), Cambridge, MA: MIT Press
 "
"