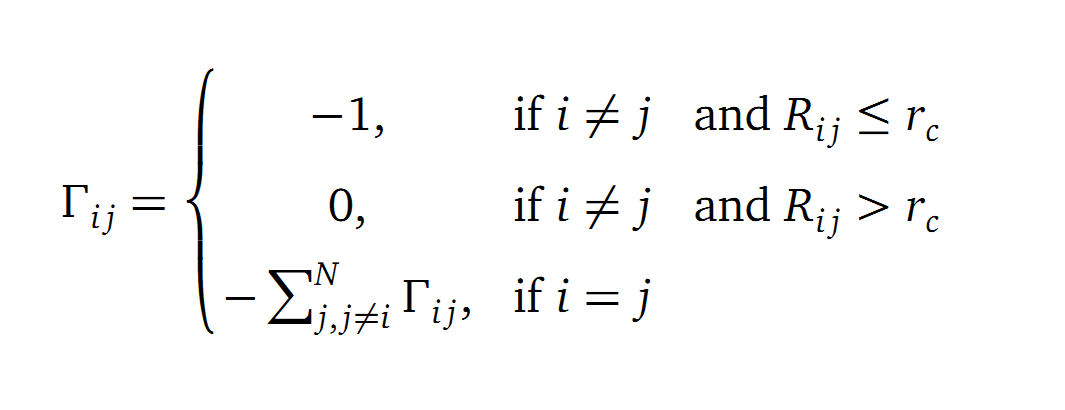Team:TU Darmstadt/Modeling GNM
From 2012.igem.org
(→Results) |
(→Gaussian network model) |
||
| Line 61: | Line 61: | ||
* TpHA2 | * TpHA2 | ||
* TpHA3 | * TpHA3 | ||
| - | + | ===Results=== | |
| + | * AroY | ||
| + | [[File: aroy_gnm.png |700px|center]] | ||
| + | * TpHA1 | ||
| + | [[File: TpHA1_gnm.png |700px|center]] | ||
| + | * TpHA2 | ||
[[File: TpHA2_gnm.png |700px|center]] | [[File: TpHA2_gnm.png |700px|center]] | ||
| - | + | * TpHA3 | |
| + | [[File: TpHA3_gnm.png |700px|center]] | ||
| + | * PnB-Esterase 13 | ||
| + | [[File: pnb_gnm.png |700px|center]] | ||
====References==== | ====References==== | ||
Revision as of 17:17, 15 September 2012
Contents |
Gaussian network model
Theory
Nearly all biologically important processes such as enzyme catalysis,ligand binding and allosteric regulation occur on a large time-scale (micro- to millisecond). A Gaussian network model (GNM) is a coarse-grained representation of a protein as an network consisting of balls and springs. In our approach, proteins are represented by balls corresponding to the CA –atom of each residue[1] . While Molecular Dynamics (MD) simulations are computational expensive, a GNM calculation only needs a few seconds.
Computation
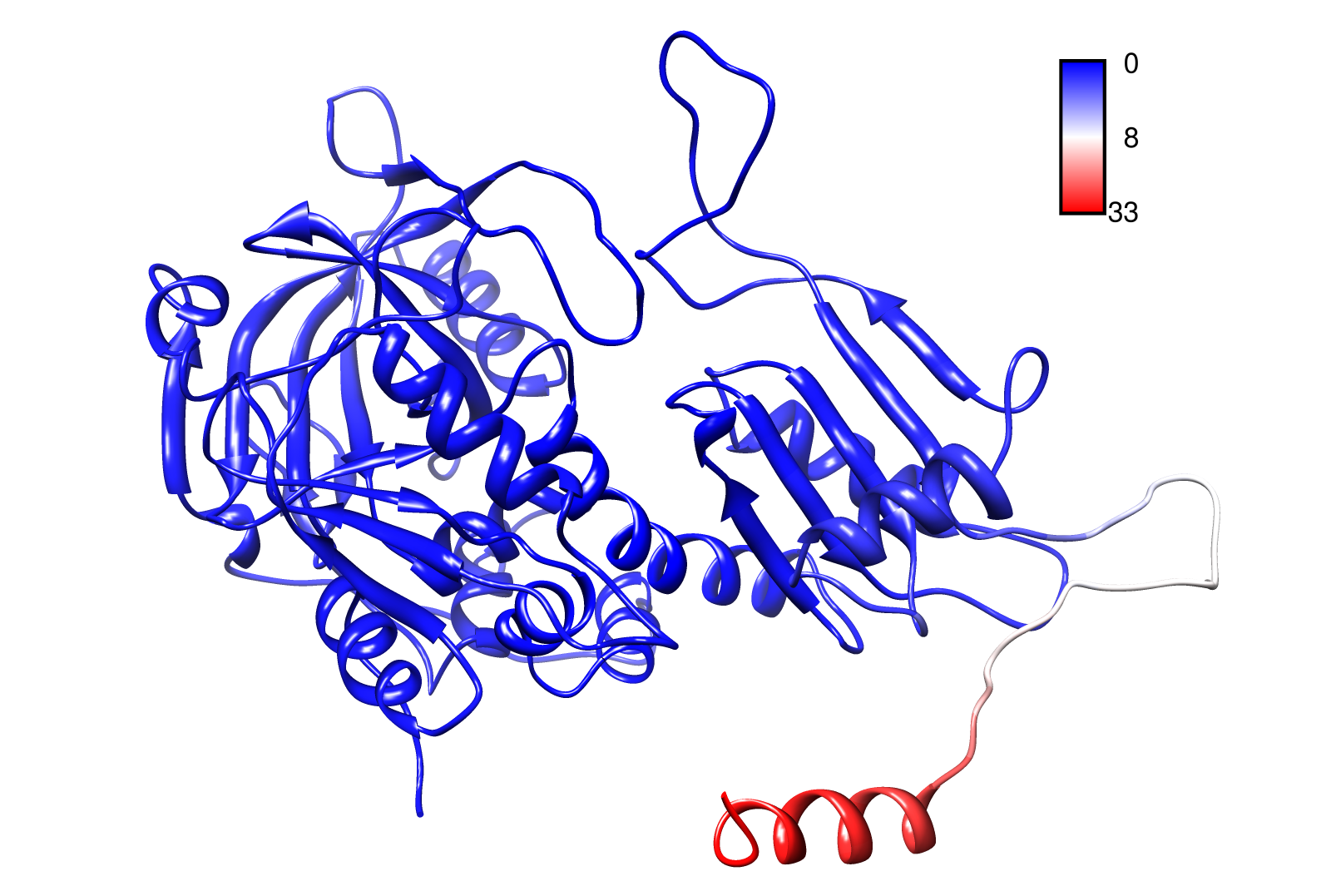
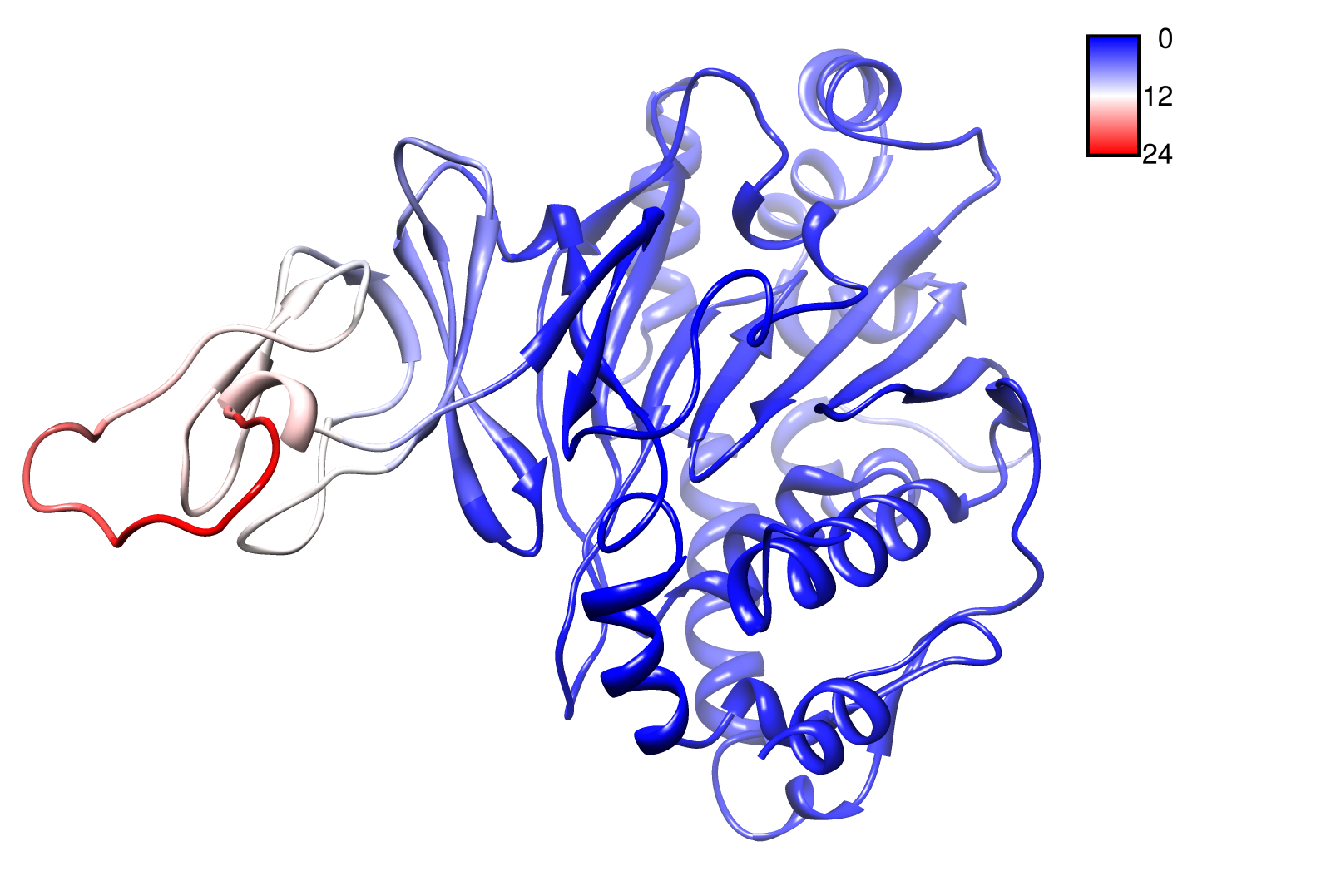
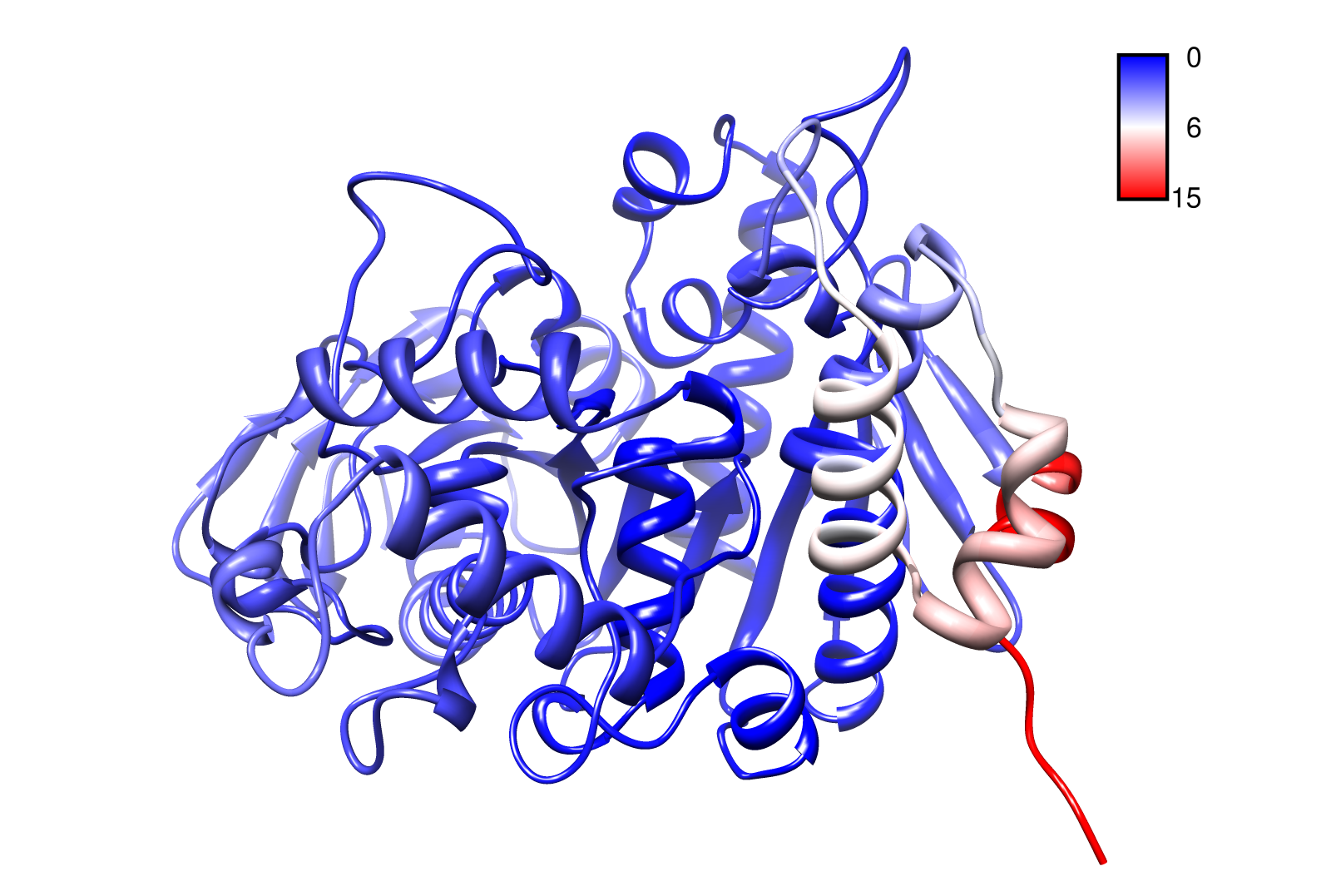
The dynamics of the structure in the GNM is described by the topology of contacts within the Kirchhoff matrix G. Thus in this network of N interacting sites, the elements of G are computed as:
where Rij is the distance between point i and j. We used Gamma as the intra CA-contact matrix. The inverse of it describes correlations between fluctuations within the proteins native state. The diagonal of the matrix is replaced by the sum of contacts of one CA-atom within the whole protein. After a singular value decomposition (SVD) we have calculated the normal modes of the protein. Slow modes describe functionally relevant residues within a biomolecule[2]. The opposite, Fast modes, represent an uncorrelated motion without significant changes in the structure.
A recent examination of the X-ray crystallographic B-factors of over 100 proteins showed that the GNM closely reproduces the experimental data [3].
Application to our Proteins
We computed the GNM in R [4] by using the BioPhysConnectoR [5] library.
- PnB-Esterase 13
- AroY
- TpHA1
- TpHA2
- TpHA3
Results
- AroY
- TpHA1
- TpHA2
- TpHA3
- PnB-Esterase 13
References
[1] A. R. Atilgan, S. R. Durell, R. L. Jernigan, M. C. Demirel, O. Keskin, and I. Bahar, “Anisotropy of fluctuation dynamics of proteins with an elastic network model.,” Biophys J, vol. 80, no. 1, pp. 505–515, Jan. 2001.
[2] C. Chennubhotla, A. J. Rader, L.-W. Yang, and I. Bahar, “Elastic network models for understanding biomolecular machinery: from enzymes to supramolecular assemblies.,” Physical Biology, vol. 2, no. 4, pp. S173–S180, 2005.
[3] I. Bahar and A. J. Rader, “Coarse-grained normal mode analysis in structural biology.,” Current Opinion in Structural Biology, vol. 15, no. 5, pp. 586–592, 2005.
[4] R. D. C. Team, “R: A Language and Environment for Statistical Computing.” Vienna, Austria, 2008.
[5] F. Hoffgaard, P. Weil, and K. Hamacher, “BioPhysConnectoR: Connecting sequence information and biophysical models.,” BMC Bioinformatics, vol. 11, p. 199, 2010.
 "
"