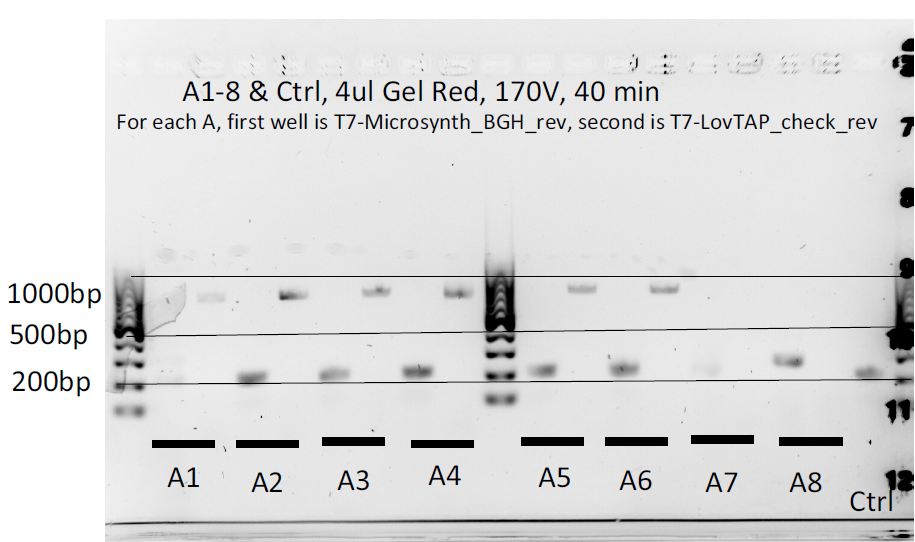
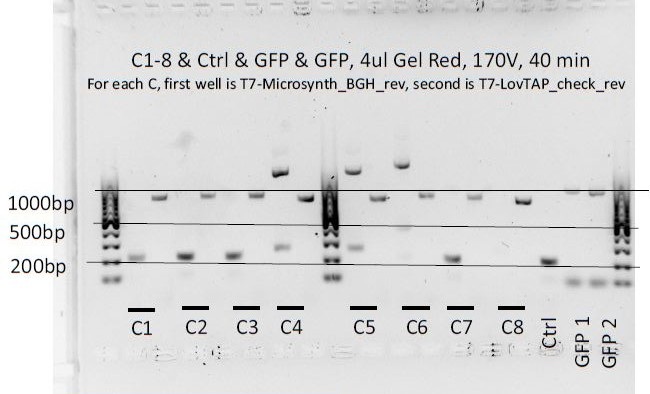
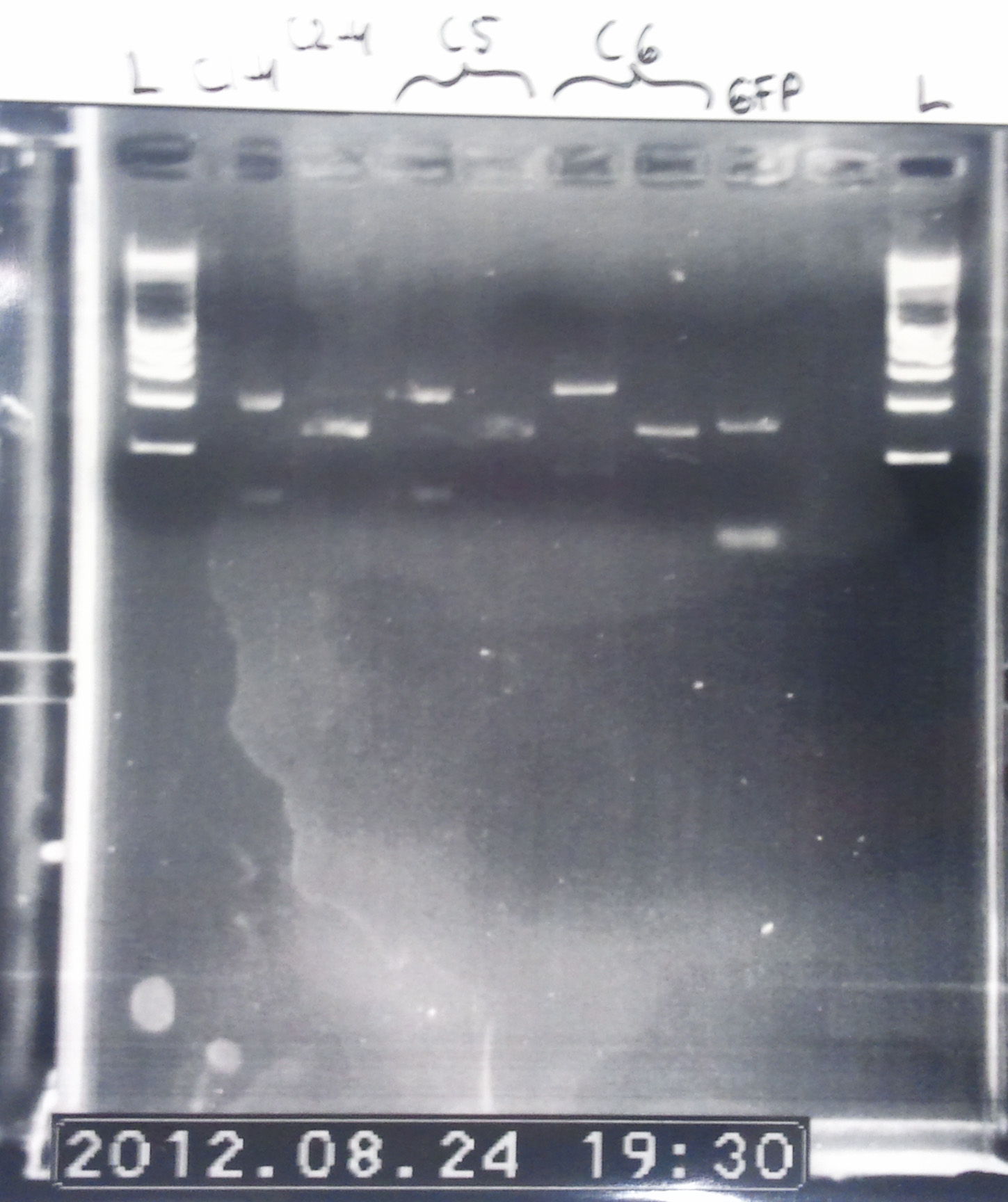
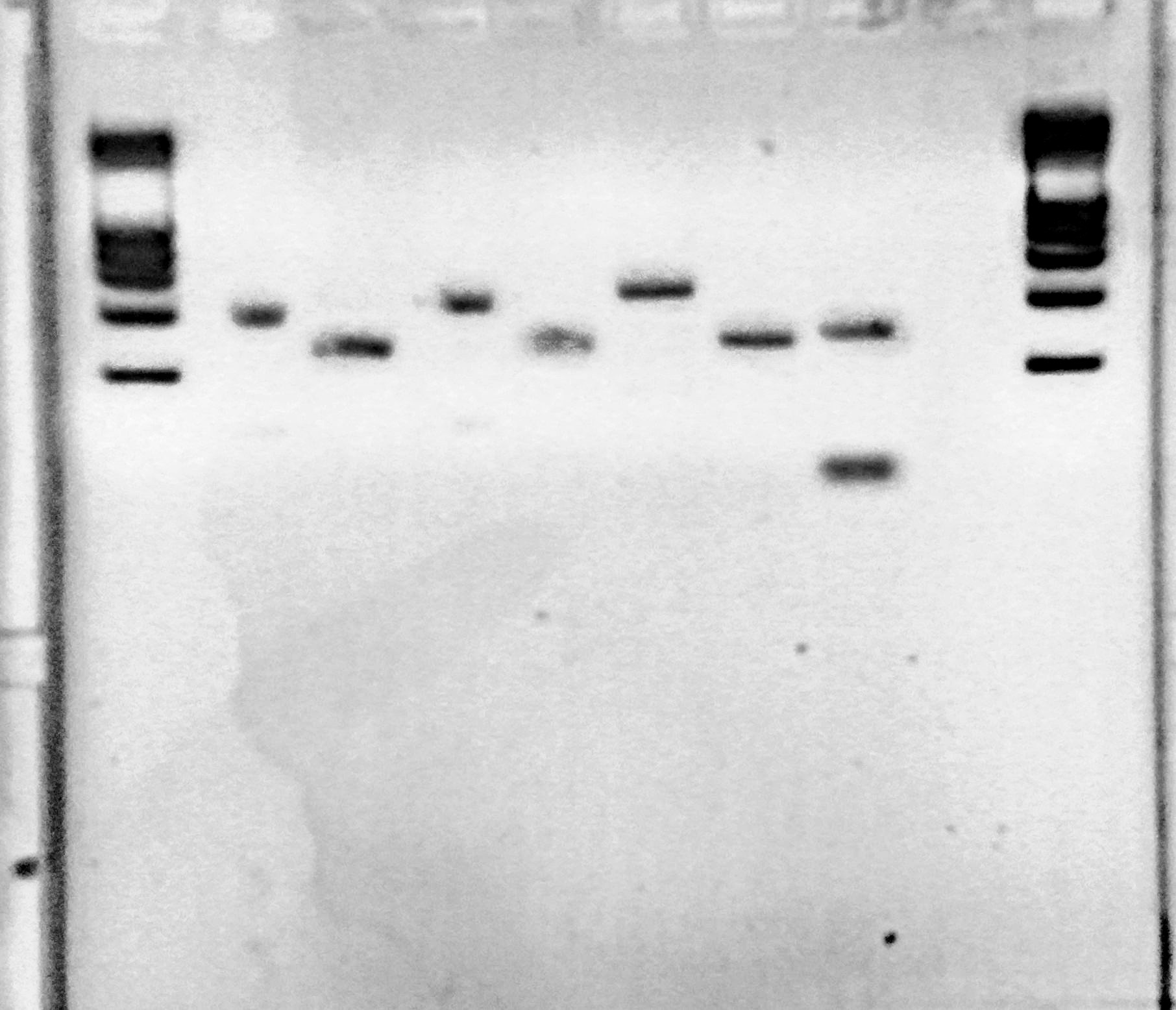
Colony PCR of pcDNA3.1(+)-LovTAP (part 2)
Protocol: PCR
PCR is a reaction that makes it possible (and relatively easy) to amplify
a certain region of DNA. The first step is the selection of that region
(and the design of the relevant primers). Primer design can be done by hand, or by
using our Primer Design Helper. Once
done, order the primers (in our case, we ordered from them [http://www.idtdna.com/ IDT]).
When you've received the primers, prepare them and make sure you've got your PCR kit (we used the "Phusion® High-Fidelity DNA Polymerase"). Start preparing your master mix, the composition for one tube is:
1X Mastermix 20μl reaction, add in this order
| Reagent | Volume [μl] |
|---|---|
| Water | Complete to total volume of 20μl |
| HF-Buffer (5x) | 4 |
| DMSO (optional) | 0.6 |
| dNTPs | 0.4 |
| Forward primer (50μM) | 0.2 |
| Reverse primer (50μM) | 0.2 |
| Template (10ng/μl) | 0.5 |
| Phusion HF polymerase | 0.2 |
Prepare one or two extra tubes-worth of reagent (you'll use some liquid on the walls of your tips).
Once you've finished, you should run the resulting products on a gel to check if everything went as planned.
Tips
- Thaw the HF-Buffer, DMSO and dNTPs before making the mastermix.
- Avoid taking the Phusion-HF polymerase out of the freezer (only take it out briefly when you need to add it).
- If the reactions have different primers and/or template, add the polymerase right after the dNTPs, split the mastermix and add the rest.
- Don't forget positive and negative controls
- Primers should have similar Tms (less than 5°C).
- Primer Tm calculation is a less exact science than it should be (just test several tools and compare their results). If you're not sure what the correct Tm is, consider using a gradient PCR.
- Avoid primers with strong secondary structures.
- PCR can introduce mutations. Don't forget to sequence your final product (this could be your final plasmid): you really don't want to lose a few weeks because of a "corrupt" plasmid.
8 colonies of each of the 3 ligation plates and 1 of the negative control plate had been dipped into Lyse'n'Go and then used as PCR template (1ul/20ul reaction).
Gel electrophoresis
Protocol: Gel Electrophoresis
Agarose concentration depends on the size of the DNA to be run. We will mostly use 1%.
VOL is the desired volume of gel in ml:
CH Lab
- Add 0.01*VOL g of agarose to a clean glass bottle.
- Pour VOL/50 ml of 50xTAE in a graduated cylinder. Fill up to VOL ml with di water.
- Add the resulting VOL ml of 1xTAE to the glass bottle with agarose.
- Microwave, at 7, the bottle (loose cap!) until it boils.
- Carefully remove bottle (can be super heated!) and check for the total absence of particles. Microwave again if needed.
- Prepare a gel box, with comb, and fill it up with the agarose solution (maybe not the whole solution is needed).
- Add 0.05 µl per ml of gel in the box of Red Gel (it's in the iGEM drawer) and stirr until disolved.
- Wait until cold and solidified.
- Carefully remove comb.
- Place the box in the electrophoresis chamber.
- Fill up the electrophresis chamber with 1x TAE buffer.
- Add blue dye to the DNA samples (6x loading buffer, that is 10 µl in 50 µl of DNA solution).
- Inject 30 µl of ladder marker in the first well (that's 1 µg of DNA).
- Inject 60 µl of each DNA solution in the other wells.
- Set voltage to 70-90 V and run for 30-40 min, or until the dye reaches the last 25% of the gel length (DNA travels from - to +).
- Place the gel under the camera, cover, turn UV on and take photos!
Preparing the ladder:
- get 1kb ladder DNA from the freezer (500 µg/ml).
- for 30 charges, 30 µl per charge, we need 900 µl:
- 60 µl of 1kb ladder DNA
- 150 µl of dye (6x loading buffer)
- 690 µl of water
BM Lab
In this lab the gels are slightly different. The total volumes for the small, the medium and the large gel are respectively 60ml, 80ml and 90ml. As we use 0.5x TAE buffer instead of 1x, we can use higher voltages (170V seems to work fine). The gel should run 20-40 minutes, not more. As the gel is thinner, load less DNA (up to ~10ul).
BM version of the gel. Colonies from the three plates were ran on it.
- Comments
We can notice a certain weird consistency in the results, though the general tendency here is not what we expected! Only colonies C4, C5 and C6 look promising, and even those have double bands. It was decided to re-run these ones on another gel later on.
Gel electrophoresis reloaded
Protocol: Gel Electrophoresis
Agarose concentration depends on the size of the DNA to be run. We will mostly use 1%.
VOL is the desired volume of gel in ml:
CH Lab
- Add 0.01*VOL g of agarose to a clean glass bottle.
- Pour VOL/50 ml of 50xTAE in a graduated cylinder. Fill up to VOL ml with di water.
- Add the resulting VOL ml of 1xTAE to the glass bottle with agarose.
- Microwave, at 7, the bottle (loose cap!) until it boils.
- Carefully remove bottle (can be super heated!) and check for the total absence of particles. Microwave again if needed.
- Prepare a gel box, with comb, and fill it up with the agarose solution (maybe not the whole solution is needed).
- Add 0.05 µl per ml of gel in the box of Red Gel (it's in the iGEM drawer) and stirr until disolved.
- Wait until cold and solidified.
- Carefully remove comb.
- Place the box in the electrophoresis chamber.
- Fill up the electrophresis chamber with 1x TAE buffer.
- Add blue dye to the DNA samples (6x loading buffer, that is 10 µl in 50 µl of DNA solution).
- Inject 30 µl of ladder marker in the first well (that's 1 µg of DNA).
- Inject 60 µl of each DNA solution in the other wells.
- Set voltage to 70-90 V and run for 30-40 min, or until the dye reaches the last 25% of the gel length (DNA travels from - to +).
- Place the gel under the camera, cover, turn UV on and take photos!
Preparing the ladder:
- get 1kb ladder DNA from the freezer (500 µg/ml).
- for 30 charges, 30 µl per charge, we need 900 µl:
- 60 µl of 1kb ladder DNA
- 150 µl of dye (6x loading buffer)
- 690 µl of water
BM Lab
In this lab the gels are slightly different. The total volumes for the small, the medium and the large gel are respectively 60ml, 80ml and 90ml. As we use 0.5x TAE buffer instead of 1x, we can use higher voltages (170V seems to work fine). The gel should run 20-40 minutes, not more. As the gel is thinner, load less DNA (up to ~10ul).
We ran the gel again in the CH lab, using only the C4, C5 and C6 colonies. Both PCR products were used for each one (they don't have the same primers).
- Gel scheme
- Lane 1: ladder
- Lane 2: C4 with T7 primer and Microsynth_BGH_rev primer
- Lane 3: C4 with T7 primer and LovTAP_check_rev primer
- Lane 4: C5 with T7 primer and Microsynth_BGH_rev primer
- Lane 5: C5 with T7 primer and LovTAP_check_rev primer
- Lane 6: C6 with T7 primer and Microsynth_BGH_rev primer
- Lane 7: C6 with T7 primer and LovTAP_check_rev primer
- Lane 8: GFP1 control
- Lane 9: nothing
- Lane 10: ladder
We observed some double bands again (for C4 and C5). Colony C6 seems to behave as expected.
Overnight culture
Protocol: Prepare Plasmid Extraction (culture for Miniprep)
- Select and number colonies on the plates.
- Prepare tubes of LB medium with the correct quantity of antibiotics (100 µg/ml for Amp, Spc or chloramphenicol).
- Amp can be found in the -20ºC freezer at Ecoli, labeled as "stock". It is 100 µg/µl, or 1000x.
- The tubes to be used are the 14 ml round bottom found in front of the iGEM drawers (Falcon). Culture with cap in the first step (loose) and close to the second step after culture.
- Touch each colony with a clean pipette tip and put it in a tube.
- Put in incubator.
The C6 colony looked correct, so it was decided to make a miniprep out of it for sequencing and other purposes. It was cultured overnight in a Falcon tube.
Ligation of purified PCR products into backbones
Protocol: Ligation
Ligation is a method of combining several DNA fragments into a single plasmid. This is often the
step following a PCR (and a PCR cleanup) or a gel extraction. You can also do a "dirty" ligation, where you follow a certain number of digestions directly by a ligation.
- Download the following spreadsheet : File:Team-EPF-Lausanne Ligation.xls
- Fill in the pink areas with the vector and fragment concentration, their size and the ratio.
- Add all the suggested ingredients order in a microcentrifuge tube, in the order they appear.
- Ligate for 2 hours at 14ºC.
- Immediately transform competent bacteria with the ligation product.
Note: This protocol hasn't been optimized for blunt-end ligation (though it might still work).
PCR products have been purified the day before.
For the melanopsin experiments:
- TNFR into pGL
- eGFP into pGL
SEAP can't be ligated yet! Digestion of pGL with MfeI required!
For LovTAP:
- Matt's PCR LovTAP into pMP
- RO into pcDNA3.1(+)
Transformation of the ligations
Protocol: Ligation
Ligation is a method of combining several DNA fragments into a single plasmid. This is often the
step following a PCR (and a PCR cleanup) or a gel extraction. You can also do a "dirty" ligation, where you follow a certain number of digestions directly by a ligation.
- Download the following spreadsheet : File:Team-EPF-Lausanne Ligation.xls
- Fill in the pink areas with the vector and fragment concentration, their size and the ratio.
- Add all the suggested ingredients order in a microcentrifuge tube, in the order they appear.
- Ligate for 2 hours at 14ºC.
- Immediately transform competent bacteria with the ligation product.
Note: This protocol hasn't been optimized for blunt-end ligation (though it might still work).
The ligation products have been used to transform bacteria. They were plated at 21.30.
Nanodrop of the cleaned-up PCR products
Protocol: DNA Concentration Measurement
- Take a 6 µl aliquote of the DNA and put back the main DNA tube in the fridge.
- Go to the room by the E.Coli lab (LBTM, not on Friday morning!) with:
- The 6 µl aliquote
- A 10 µl pipet
- Optionally, the buffer you used for DNA elution (there might be some next to the machine).
- The machine is the NanoDrop Spectrophotometer.
- On the computer, click on "Nucleic Acid".
- Put a 2 µl drop of (nuclease-free) water on the machine's tip as you are asked to and measure.
- Clean tips (both sides) with a quarter of tissue.
- Add 2 µl of the buffer you use and click on "Blank".
- Clean tips (both sides).
- Add 2 µl of your DNA sample and click "Measure".
- Clean tips (both sides) with a tissue.
- Take 2 measurements per sample (for averaging).
- Print the report when you are done
- Click on exit.
The important numbers are:
- 260/280 ratio, must be > 1.8
- 260/230 ratio, must be > 2 (too big, > 2.5? , might mean too much salts)
- Of course the DNA concentration.
Cleaned-up PCR products (two different RO digestions, GFP, TNFR, SEAP, LovTAP PCR'd by Matt) from the day before were checked on the Nanodrop. The DNA yield was low as always (from 2 ng/µl to 20 ng/µl).
VP16 antibody efficiency check
Protocol: Western Blot
Gel Ingredients (choose percentage according to the size of the protein)
| 4-40 kDA | 20% |
| 12-45 kDA | 15% |
| 10-70 kDA | 12.5% |
| 15-100 kDA | 10% |
| 25-200 kDA | 8% |
| Separating gel | |
| Gel percentage | 7.5 % |
| 30% Polyacrylamide | 10 mL |
| 1.5M Tris (pH 8.8) | 10 mL |
| 10% Ammonium persulfate | 0.4 mL |
| 10% SDS | 0.4 mL |
| TEMED | 0.038 mL |
| H2O | 19.2 mL |
| Total volume | 40 mL |
| Stacking gel | |
| Gel percentage | 5 % |
| 30% Polyacrylamide | 1.36 mL |
| 1M Tris (pH 6.8) | 1 mL |
| 10% Ammonium persulfate | 0.08 mL |
| 10% SDS | 0.08 mL |
| TEMED | 0.008 mL |
| H2O | 5.44 mL |
| Total volume | 8 mL |
Preparing Protein Samples
1. Centrifuge around 5 million cells (of any volume) at 2,500 rpm for 10 min.
2. Discard the supernatant with a vacuum pump.
3. Resuspend the cell pellet with 1x PBS and centrifuge it at 2,500 rpm for 10 min.
4. Discard the supernatant with a vacuum pump.
5. Add appropriate amount of lysis buffer depending on the pellet size (for a 20 mg pellet, 150 µl of IP lysis buffer).
6. Keep the lysed sample on ice for 10 min - flick every 3 minutes.
7. Add 3x SDS lysis buffer (for a 20 mg pellet, 75 µl).
8. Incubate the sample for 5 minutes at 95 degrees, to denature proteins.
Preparing loading samples
1. Load the ladder (7 µl is the recommended volume).
2. Complete sample volume to 50 µl.
3. Load the samples.
I. SDS Gel electrophoresis
1. Prepare the separating and stacking gel solutions without APS and TEMED.
2. Add APS and TEMED to the separating gel solution only when the SDS kit is ready to be used, they are time-sensitive. Move the solution inside of the setup. Add some distilled water on top of it.
3. After 20-30 mins, remove the water and check whether the gel has solidified. Don't move to the next step until it does.
4. Add TEMED to the stacking gel solution, pour it on top of the solidified separating gel.
5. Insert a stack carefully and leave it for 20-30 mins.
6. Take the stack out and fill the kit with SDS loading buffer.
7. Load the samples.
8. Add more loading buffer, set the voltage to 80 Volts. Leave for 1.5 hours.
II. Membrane transfer
1. Prepare a membrane transfer kit.
2. Take the gel out of the SDS kit and put it on the membrane paper.
3. From bottom to top, assemble the components in the following order: 1) Sponge - 2) Blot paper - 3) Membrane - 4) Gel (Pour some M-transfer buffer on the gel) - 5) Blot paper again - 6) Sponge again.
4. Close the sandwich, set the voltage to 20 V. Leave for 30 mins - 1 hour.
5. Discard the gel. Leave the membrane in 5% skim milk with 30ml of TBST buffer (blocking buffer, to achieve the 5%, add 1.5 g of skim milk powder to the buffer) for one hour.
III. Antibody tagging
1. Discard the blocking buffer, leave only 5ml of it. Add primary antibody with a ratio of 1:1000 or 1:2000 (5 µl of antibody in 5 ml of buffer gives 1:1000)
2. Leave the mix overnight at 4 °C.
3. Wash 3 times with 1x TBST (5 minutes on shaker for every wash).
4. Dilute the secondary antibody (for example, goat anti-rabbit antibody) to 1:2000 in 5% skim milk buffer. Add it. Leave at room temperature for 2 hours.
5. Wash 3 times with 1x TBST (5 minutes on shaker for every wash).
6. Reveal the protein bands in the dark room.
I. VP16 sample preparation
1. VP16 has been aliquoted and stocked at -80°C again. (There are two samples of VP16, labeled "Good" and "Bad". "Good" means it has been preserved at -80°C at all times, "Bad" means it has been stored at room temperature for a while upon delivery arrival. It may have denatured.)
2. Diluted the VP16 sample with the 1x TBST solution and made a 10 µg/µl solution of VP16 sample.
3. Loaded 5 µg, 10 µg, 15 µg of VP16 (a "Good" and "Bad" for each one, totaling 6 samples).
II. CHO cell lysate with VP16 1. CHO cells have been lysed and mixed with VP16. 2. Made a CHO cell concentration gradient - 20/30/40 µl with the "Good" VP16 sample.
- Lanes 1 - 10
- 1. Ladder = 7 µl
- 2. 20 µl of CHO cell lysis + 1 µl of VP16 (10 µg) + 29 µl of SDS lysis buffer = 50 µl total
- 3. 30 µl of CHO cell lysis + 1 µl of VP16 (10 µg) + 19 µl of SDS lysis buffer = 50 µl total
- 4. 40 µl of CHO cell lysis + 1 µl of VP16 (10 µg) + 9 µl of SDS lysis buffer = 50 µl total
- 5. 24.5 µl of SDS lysis buffer + 0.5 µl of VP16 (5 µg) = 25 µl total ("Good")
- 6. 24 µl of SDS lysis buffer + 1 µl of VP16 (10 µg) = 25 µl total ("Good")
- 7. 23.5 µl of SDS lysis buffer + 1.5 µl of VP16 (15 µg) = 25 µl total ("Good")
- 8. 24.5 µl of SDS lysis buffer + 0.5 µl of VP16 (5 µg) = 25 µl total ("Bad")
- 9. 24 µl of SDS lysis buffer + 1 µl of VP16 (10 µg) = 25 µl total ("Bad")
- 10. 23.5 µl of SDS lysis buffer + 1.5 µl of VP16 (15 µg) = 25 µl total ("Bad")
Stocked this in -4°C refrigerator, will tag antibodies on Monday.
 "
"