Team:UNITN-Trento/Project/CrustAway
From 2012.igem.org
| Line 20: | Line 20: | ||
<body> | <body> | ||
| + | <!--[if lte IE 8]> | ||
| + | <style type="text/css"> | ||
| + | body { | ||
| + | filter:blur(add=false, direction=270 strength=10); | ||
| + | -ms-filter:blur(add=false, direction=270 strength=10); | ||
| + | background-image: none; | ||
| + | } | ||
| + | #bannerImage { | ||
| + | display: none; | ||
| + | } | ||
| + | #header { | ||
| + | display: none; | ||
| + | } | ||
| + | #canvas { | ||
| + | display: none; | ||
| + | } | ||
| + | #footer { | ||
| + | display: none; | ||
| + | } | ||
| + | </style> | ||
| + | <h2 style="font-family: 'Open Sans'; line-height: 40px; color:#ffae00; padding:0.5em; font-size:30pt !important; text-align: center; width: 900px; margin: 100px auto;">It appears you're using Internet Explorer. This website does not work at his best on older browsers. Please use a modern one like <a href="http://www.google.com/chrome">Chrome</a>, <a href="http://www.mozilla.org/en-US/firefox/new">Firefox</a> or <a href="http://www.apple.com/safari">Safari</a> to enjoy the full experience. </h2> | ||
| + | <![endif]--> | ||
| + | |||
| + | <noscript> | ||
| + | <style type="text/css"> | ||
| + | body { | ||
| + | filter:blur(add=false, direction=270 strength=10); | ||
| + | -ms-filter:blur(add=false, direction=270 strength=10); | ||
| + | background-image: none; | ||
| + | } | ||
| + | #bannerImage { | ||
| + | display: none; | ||
| + | } | ||
| + | #header { | ||
| + | display: none; | ||
| + | } | ||
| + | #canvas { | ||
| + | display: none; | ||
| + | } | ||
| + | |||
| + | #footer { | ||
| + | display: none; | ||
| + | } | ||
| + | </style> | ||
| + | |||
| + | <h2 style="font-family: 'Open Sans'; line-height: 40px; color:#ffae00; padding:0.5em; font-size:30pt !important; text-align: center; width: 900px; margin: 100px auto;">It appears you have JavaScript disabled. Our Wiki needs it for you to enjoy the full experience. Please enable Javascript and refresh the page.</h2> | ||
| + | </noscript> | ||
<div id="bannerImage" class="content-fill" style="overflow: hidden; position: absolute; height: 400px;"></div> | <div id="bannerImage" class="content-fill" style="overflow: hidden; position: absolute; height: 400px;"></div> | ||
Revision as of 11:44, 19 September 2012
Crust Away
INTRODUCTION
Have you ever noticed that ugly black crust found on precious monuments and statues? Here in Italy, we surely have it, and we’d like to find a way to return our monuments to their natural, beautiful state.

Our idea is to create a bioremediation kit to help every sculptor, restorer, marble cutter or anyone interested in removing that ugly and harmful black crust layer from their precious calcareous stones. A lot of chemical and mechanical approaches are already off the shelf, and someone also exploited in the past Sulfur Reducing Bacteria (SRB) to eliminate the trapping gypsum matrix (Capitelli et al. 2007).
However all of these methods show some weak points:
- Chemical and mechanical methods are too invasive and they risk to damage the underlying and precious marble surface; We have met with experts in the restoration field and learned about the criteria to define a good restoration.
- Chemical methods have also health related risks. Workers, artists, restorers too often use chemicals to clean marble stones in unsafe conditions. We have met with one of the many local artists and reported our impressions on his methods to clean marble in the Art & Science section of our Wiki.
- Natural SRB, which have been used in a few cases, instead need anaerobic conditions and they constitutively express the enzymes required for sulphur reduction, thus not leaving the choice to the operator to control the rate and amount of sulphur reduced.
- Work in AEROBIC conditions: this is a breakthrough in the field of SRB!
- Should be CONTROLLED and MODULATED as needed.
- Should NOT be INVASIVE and work selectively against the gypsum matrix without touching the calcareous surface.
- Should be CHEAP and EASY TO APPLY. There will be no more need of expensive chemical and trained workers that have to work many hours to clean our statues and monuments.
To summarize, with our biological system we want to mix the strengths of all the available methods and eliminate their weak points to propose an infallible and very safe method to clean precious marble pieces!
How does the black crust form?
One of the main factors in the formation of the black crust is the atmospheric pollution. The burning process of fossil fuels leads to an increase in the concentration of some acid gases in the atmosphere. In particular SO2 when reacts with water induces the transformation of calcite (CaCO3, present in the stone substrate) into gypsum (CaSO4 ·2H2O), which precipitates with inclusions of carbon particulate matter. Smog particles are also able to absorb gas pollutants on their surfaces, resulting in “dry deposition” of pollutant.
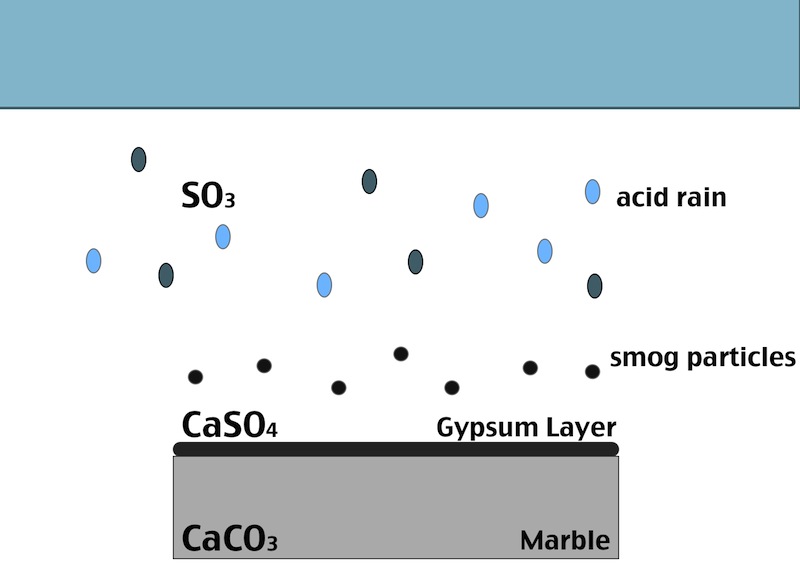
Black crust are usually found on areas of the stone sheltered from rainfall, although still in presence of capillary water flowing through the pores. Calcium ions migrate to the surface of the stone during gypsum formation, leading to formation of cavities beneath the stone that weakens the stone’s integrity. The degradation process indeed affects both the stone conservation and appearance:
- When the formed gypsum is washed away it takes some of the stone particles with it, causing at first loss of detail, but eventually leading, again, to a loss of structural integrity.
- In areas sheltered from rainwater, though, gypsum crust remain. When combined with particulate matter from the atmosphere, gypsum creates the so called black crust.
OUR METHOD
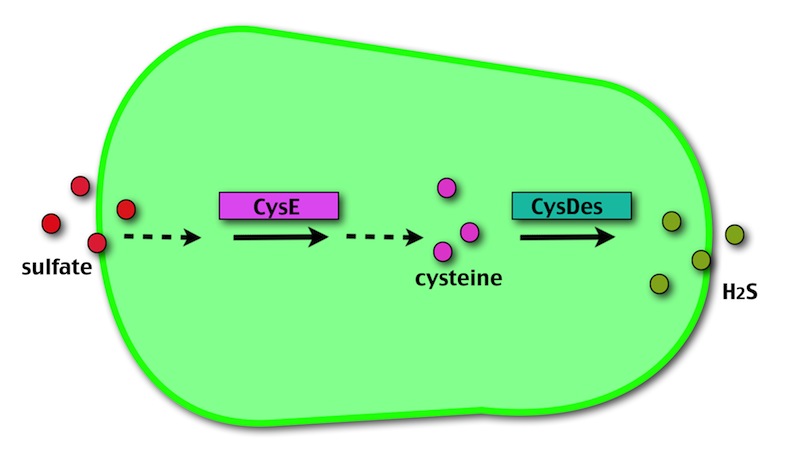
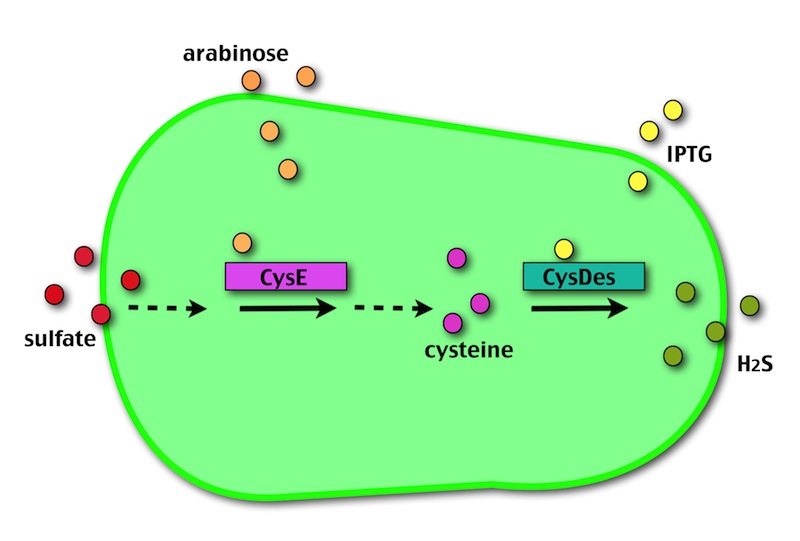
Our method exploits an engineered E. coli to reduce aerobically the sulphuric gypsum layer in a controlled and modulated manner. In 2001 the Keasling group has engineered bacteria to reduce sulphate aerobically and precipitate metals from water taking advantage of the production of sulfidric acid as one of the bioproducts of sulphur reduction. We took inspiration from this work and developed two new BioBrick devices directed to overproduce cysteine and then convert it to sulphide with the ultimate goal of dissolving the sulphate layer deposited on the blackened stones.
Who are the players?
- CysE is a Serine acetyltransferase that mediates conversion of L-serine to a precursor of L-cysteine in E.coli. More precisely we have used a mutant CysE (M256I) that has enhanced activity in that its enzymatic activity is less sensitive to feedback inhibition by cysteine.
- CysDes is a Cysteine Desulfhydrase, first isolated in Treponema denticola, an anaerobic organism involved in periodontal diseases. It is an aminotransferase that converts cysteine into pyruvate, ammonia, and hydrogen sulphide.
How do we control the expression of our devices?

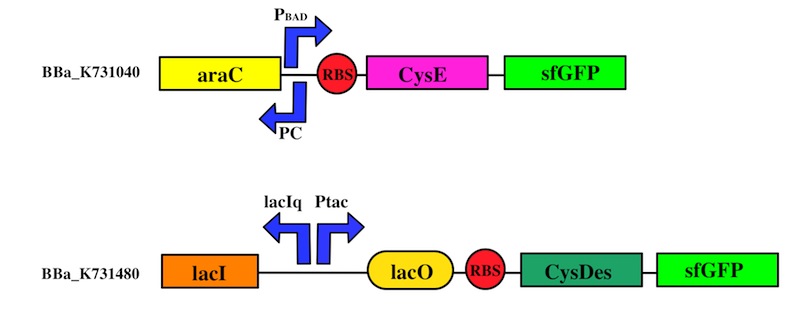
The expression of CysE is controlled by an arabinose inducible cassette (our part BBa_K731201). Our composite device (BBa_K731030) is composed by araC –pBad, and strong RBS followed by M256I CysE.
The pBAD promoter is activated in the presence of L-arabinose. L-arabinose binds to the AraC protein and inactivates its inhibitory function, allowing the RNA polymerase to recognize the pBAD promoter and start the transcription of CysE.
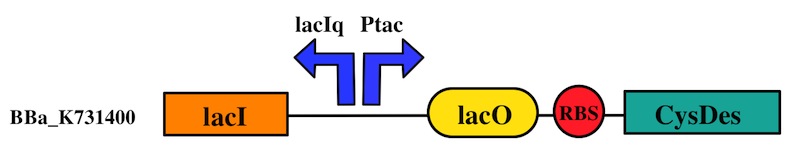
CysDes was placed downstream of an IPTG inducible cassette (our part BBa_K731300). The expression of CysDes is therefore controlled by a system composed of: LacI (repressor protein) and a lacIq promoter followed by a strong hybrid promoter (Ptac), a Lac Operator and a strong RBS. Addition of IPTG inactivates the LacI repressor, thus allowing the expression of our gene of interest CysDes.
The possibility of controlling simultaneously the expression of our two devices (i.e. by adding arabinose and/or IPTG) makes our system more SAFE, CONTROLLABLE and MODULAR.
How do we test the expression of our enzymes?
We used the Gibson assembly method to create two sfGFP-tagged devices that were useful reporter to verify the expression of our genes.
RESULTS
CysE
CysE is the black crust worst nightmare.
CysE catalyses the activation of L-serine by acetyl-CoA. The product, O-acetyl-L-serine, is a precursor for cysteine.
When much cysteine is produced, E. coli needs more sulfate, resulting in the assimilation of that found in the black crust!
Pinker is better!
As cysteine is also secreted because of CysE, assessing cysteine presence in the culture can help us analyze if and how our Part is working. We tested cysteine production of our cultures with ninhydrin, a small organic compound that reacts with different alpha-aminoacids at different pH. In the presence of cysteine at low pH the solution turns pink within few minutes. The reaction between cysteine and ninhydrin at low pH also gives a characteristic UV-VIS spectrum with an absorbance maximum peak at 560 nm.
Cells transformed with CysE and induced with 5 mM arabinose were assayed with ninhydrin along with NEB10b cells, also induced, and left to grow overnight. Samples taken before induction served as controls.
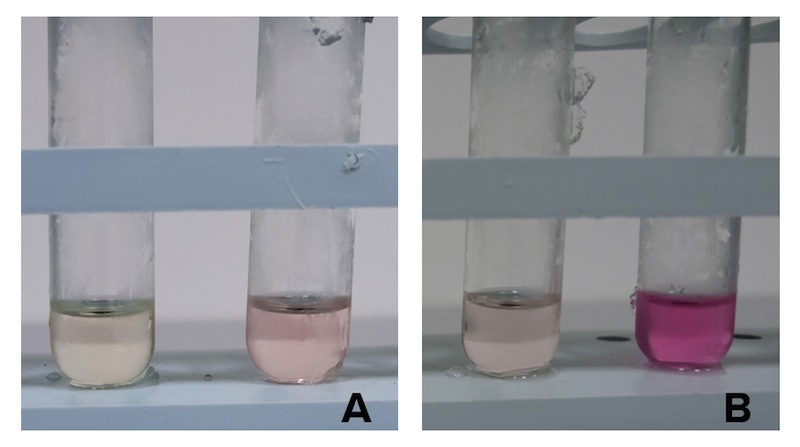
Caption: A. NEB10b cells assayed with ninhydrin. Sample before induction on the left, after induction and overnight growth. B. Cells transformed with CysE assayed with ninhydrin. Sample before induction on the left, after induction and overnight growth.
The pink pinkness of the culture with our construct is confirming that it’s working. To quantify how much cysteine was produced a standard curve was built using different concentration of cysteine.
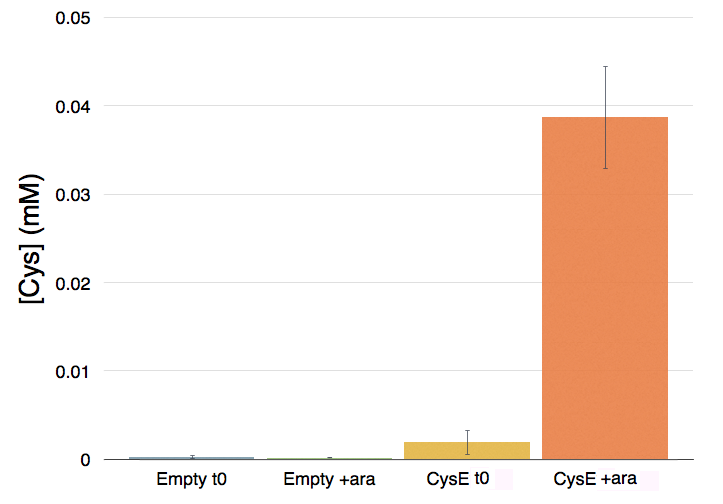
Caption: Cysteine production of samples shown in the figure above. From left to right: NEB10b before induction, NEB10b after induction and overnight growth, CysE before induction, CysE after induction and overnight growth.
To have a better way to control how the protein is expressed, we used two version of the MOPS minimal medium: one with glucose as the carbon source (MOPS B), the other with glycerol (MOPS A). As stated in “Our method”, CysE transcription should be diminished in presence of glucose, even if arabinose is present. In glycerol, this second level of control _ by glucose is bypassed.
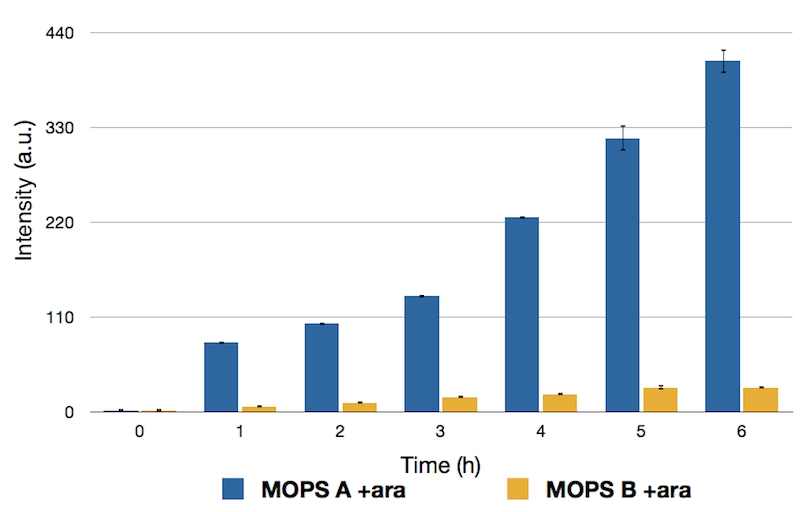
Caption: Emission Peaks measured for CysE-sfGFP after induction. Measures in MOPS A are shown in blue, measures in MOPS B are shown in yellow.
As we expected, expression in gycerol when cells are induced is way higher than in glucose, which still is not zeroed.
In glycerol, thus, cells are producing a lot of CysE. To assess if and how CysE production is affecting cell growth, we perfomed serial dilutions at 8h after induction.
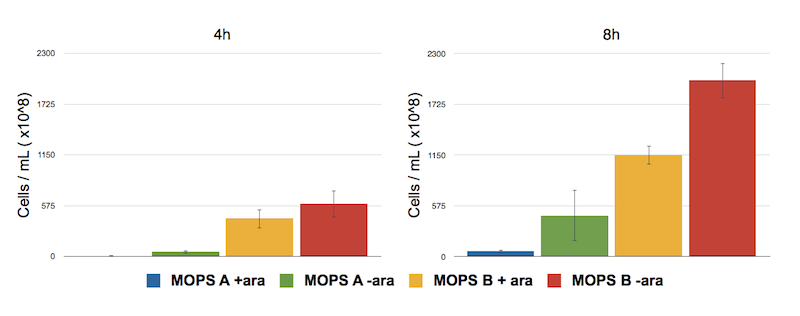
Caption: Serial Dilution of CysE containing cells performed at 4 h and 8 h. From left to right samples are: CysE in MOPS A induced, CysE in MOPS A not induced, CysE in MOPS B induced, CysE in MOPS B not induced. Cell number was calculated with the following formula: Cells/mL = (# colonies counted)*(dilution factor)/(mL of culture plated).
The results confirm our fear, showing that when cells are induced in MOPS A their growth is eventually impaired. Non induced cells in MOPS A have an higher number, which is still lower than that of cells grown in glucose, the preferred carbon source.
Now that we know how cells behave in presence of our construct, we want to know if they are actually producing what we’re interested in: cysteine. The ninhydrin assay was applied to induced and not induced cultures grown overnight both in MOPS A and MOPS B.
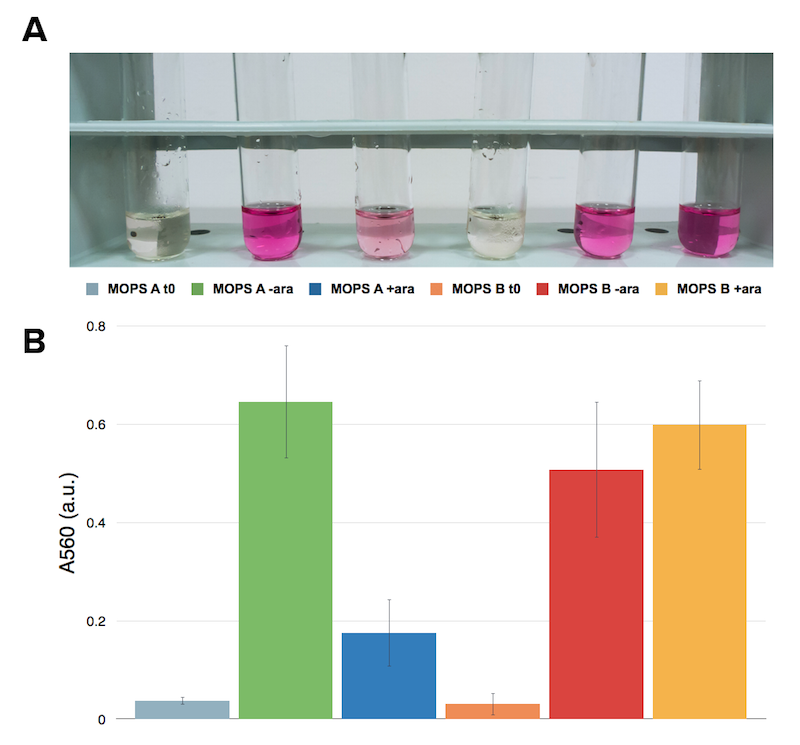
Caption: Ninhydrin assay on cell cultures of the CysE containing strain. A representative replicate is shown. From left to right samples are: CysE in MOPS A before induction, CysE in MOPS A not induced grown overnight, CysE in MOPS A induced grown overnight, CysE in MOPS B before induction, CysE in MOPS B not induced grown overnight, CysE in MOPS B induced grown overnight.
When cells are induced in glycerol they show very high expression levels, but few cells can cope with this pressure. Even if each of these few cells is actually producing much CysE, it doesn’t have the capabilities to sustain such high cysteine production, which is not achieved in a single step reaction (i.e. a bottleneck is reached with other enzymes). When cells in glycerol are not induced, CysE production lessens, allowing more cells to survive and determining higher cyteine production. In glucose the situation is opposite: we have many cells, but low expression due to glucose presence itself. All these cells are producing low amounts of CysE, but their growth is less affected, resulting in high cysteine production.
To continue with our tests, we selected MOPS B as our medium of choice, since cysteine production seems more reliable. Additionally, considering we are working on surfaces, a high number of cells is preferred.
APPLICATION
THE CRUSTANATOR: an home made acid rain simulator.
As we started collecting samples of dirty marbles we realized that it was important to test our bacteria to have an homogenous surface of black crust to be removed that was surely composed of a gyspsum layer.
Inspired by the work of a group of researchers at the University of Belfast an at the University of Oxford (M. Gomez-Heras, B.J. Smith and H.A. Viles ) we decided to build our own acid rain simulation chamber. For a detailed protocol on how to build your own Crustonator you can check our protocol section.
By using our labmade acid rain simulator we were able to successfully recreate the black crust on:
- 2 small white marbles from Carrara (20x20 cm)
- 2 small yellow marbles from Vicenza (20x20 cm)
- 1 big Carrara marble piece (50x30 cm)
The pieces were immersed partially in distilled water and subjected to three 72 hours cycles of exposure to a saturated atmosphere of sulfourus acid. At the beginning of each cycle a fine powder of charcoal was injected into the chamber with compressed air.
After the treatment the box was opened and we happily observed the formation of a gypsum layer in top of the stones and on the surface of the water. The black crust formed with better results on the small pieces probably due to chamber ventilation, direction of the ashes jet, amount of water used and marble piece dimensions. A small amount of the crust was scratched and looked under the microscope to confirm the presence of gypsum crystals.
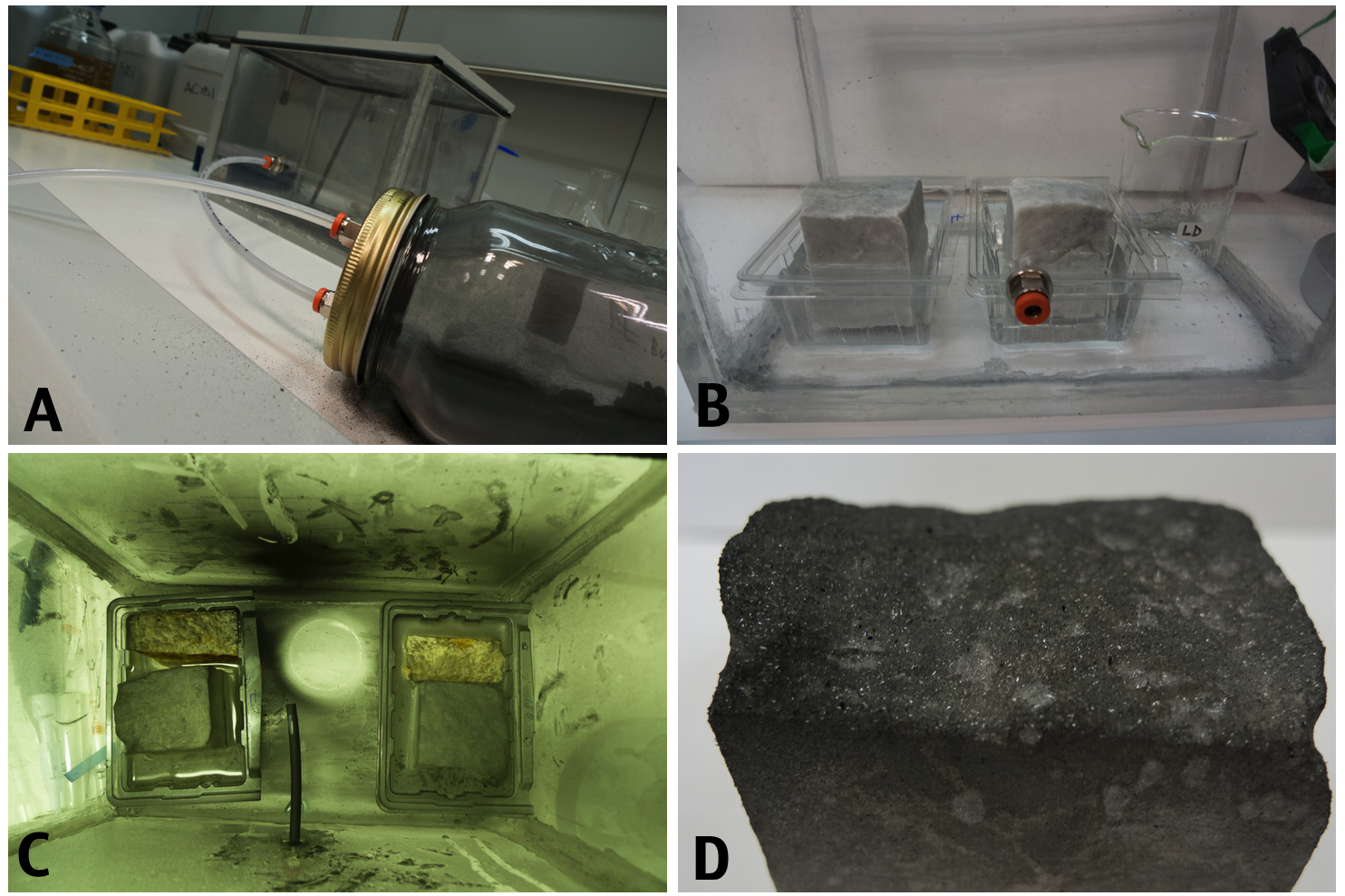
The Crustanator: A small laboratory device was built to simulate the acid rain and pollutant on the stones (A) In a tightly sealed plexiglass box, connected to a sparate chamber containg charcoal, were placed two marbles pieces from Carrara and two yellow marbles from Vicenza. The calcareous stones were partially immersed in water (B) and subjected to a saturated atmosphere of SO3 mixed with small particles of charcoal that were sprayed every 73 hours in the chamber (C). After 216 hours the box was opened and a black crust was observed on the top layer of the marble.
The blackened stones are ready to be cleaned by our bacteria!
 "
"