Team:TU Munich/Project/Thaumatin
From 2012.igem.org
| Line 27: | Line 27: | ||
===The molecular and physiological effects of thaumatin=== | ===The molecular and physiological effects of thaumatin=== | ||
| - | The '''sweet taste receptor''' is a heterodimeric receptor composed of ''T1R2'' (also TAS1R2) and ''T1R3'' (also TAS1R3) subunits. The large amino-terminal domains (NTD) of the T1R2 and T1R3 subunits have shown to be responsible for the primary ligand binding | + | The '''sweet taste receptor''' is a heterodimeric receptor composed of ''T1R2'' (also TAS1R2) and ''T1R3'' (also TAS1R3) subunits. The large amino-terminal domains (NTD) of the T1R2 and T1R3 subunits have shown to be responsible for the primary ligand binding [[http://www.ncbi.nlm.nih.gov/pubmed?term=22450161 Maîtrepierre et al., 2012]]. In addition these receptors have a transmembrane heptahelical domain. T1R receptors belong to the family of ''class C G-Protein coupled receptors'' (GPCRs), which in this case means that through ligand binding an elevation of the cAMP concentration in the taste buds is induced [[http://www.ncbi.nlm.nih.gov/pubmed?term=19489607 Ide et al., 2009], [http://www.ncbi.nlm.nih.gov/pubmed?term=15087236 Ozeck et al., 2004]]. As a result a decrease in the intracellular cAMP accumulation is measured. Released calcium (Ca2+) seems to be another independent second messenger within the transduction of the taste response (downstream of taste receptors) [[http://www.ncbi.nlm.nih.gov/pubmed?term=16510847 Trubey et al., 2006]]. |
However, not only sucralose or other sugars can bind with the NTDs of the sweet taste receptor, but also thaumatin [[http://www.ncbi.nlm.nih.gov/pubmed?term=19489607 Ide et al., 2009]]. It seems to have a longer lasting and stronger effect than sucralose. | However, not only sucralose or other sugars can bind with the NTDs of the sweet taste receptor, but also thaumatin [[http://www.ncbi.nlm.nih.gov/pubmed?term=19489607 Ide et al., 2009]]. It seems to have a longer lasting and stronger effect than sucralose. | ||
| Line 37: | Line 37: | ||
Alternatively one could have used a fusion product of prothaumatin and the Mat-alalalalalpha-factor to achieve secretion. | Alternatively one could have used a fusion product of prothaumatin and the Mat-alalalalalpha-factor to achieve secretion. | ||
| - | Preferable seems to be the natural preprothaumatin, because of the expected higher yield | + | Preferable seems to be the natural preprothaumatin, because of the expected higher yield [[http://www.ncbi.nlm.nih.gov/pubmed?term=21636903 Masuda et al., 2011]] and the possibility that the pre-sequence is necessary for the correct procession [[http://www.ncbi.nlm.nih.gov/pubmed?term=17897626 Ide et al., 2007]]. A similar construct was used by the Kyoto University (Ide ''et al.'', submitted) in ''Pichia pastoris'' with a the ''pPIC6α expression vector'' with a high yield (especially with the preprothaumatin I gene and without the α-factor secretion signal). |
To achieve the highest possible yield, we optimized the original gene sequence for best yeast codon usage (via GeneArt® GeneOptimizer®). | To achieve the highest possible yield, we optimized the original gene sequence for best yeast codon usage (via GeneArt® GeneOptimizer®). | ||
| Line 67: | Line 67: | ||
---- | ---- | ||
| - | *[Ide et al., 2007] Ide, N., Masuda, T., and Kitabatake, N. (2007). Effects of pre- and pro-sequence of thaumatin on the secretion by | + | *[[http://www.ncbi.nlm.nih.gov/pubmed?term=17897626 Ide et al., 2007]] Ide, N., Masuda, T., and Kitabatake, N. (2007). Effects of pre- and pro-sequence of thaumatin on the secretion by ''Pichia pastoris''. ''Biochem Biophys Res Commun'', 363(3):708–14. |
*[[http://www.ncbi.nlm.nih.gov/pubmed?term=19489607 Ide et al., 2009]] Ide, N., Sato, E., Ohta, K., Masuda, T., and Kitabatake, N. (2009). Interactions of the sweet-tasting proteins thaumatin and lysozyme with the human sweet-taste receptor. ''J Agric Food Chem'', 57(13):5884–90. | *[[http://www.ncbi.nlm.nih.gov/pubmed?term=19489607 Ide et al., 2009]] Ide, N., Sato, E., Ohta, K., Masuda, T., and Kitabatake, N. (2009). Interactions of the sweet-tasting proteins thaumatin and lysozyme with the human sweet-taste receptor. ''J Agric Food Chem'', 57(13):5884–90. | ||
*[Lee et al., 1988] Lee, J. H., Weickmann, J. L., Koduri, R. K., Ghosh-Dastidar, P., Saito, K., Blair, L. C., Date, T., Lai, J. S., Hollenberg, S. M., and Kendall, R. L. (1988). Expression of synthetic thaumatin genes in yeast. ''Biochemistry'', 27(14):5101–7. | *[Lee et al., 1988] Lee, J. H., Weickmann, J. L., Koduri, R. K., Ghosh-Dastidar, P., Saito, K., Blair, L. C., Date, T., Lai, J. S., Hollenberg, S. M., and Kendall, R. L. (1988). Expression of synthetic thaumatin genes in yeast. ''Biochemistry'', 27(14):5101–7. | ||
*[[http://www.ncbi.nlm.nih.gov/pubmed?term=22450161 Maîtrepierre et al., 2012]] Maîtrepierre, E., Sigoillot, M., Le Pessot, L., and Briand, L. (2012). Recombinant expression, in vitro refolding, and biophysical characterization of the n-terminal domain of t1r3 taste receptor. ''Protein Expr Purif'', 83(1):75–83. | *[[http://www.ncbi.nlm.nih.gov/pubmed?term=22450161 Maîtrepierre et al., 2012]] Maîtrepierre, E., Sigoillot, M., Le Pessot, L., and Briand, L. (2012). Recombinant expression, in vitro refolding, and biophysical characterization of the n-terminal domain of t1r3 taste receptor. ''Protein Expr Purif'', 83(1):75–83. | ||
| - | *[Masuda et al., 2011] Masuda, T., Ohta, K., Mikami, B., and Kitabatake, N. (2011). High-resolution structure of the recombinant sweet-tasting protein thaumatin i. ''Acta Crystallogr Sect F Struct Biol Cryst Commun'', 67(Pt 6):652–8. | + | *[[http://www.ncbi.nlm.nih.gov/pubmed?term=21636903 Masuda et al., 2011]] Masuda, T., Ohta, K., Mikami, B., and Kitabatake, N. (2011). High-resolution structure of the recombinant sweet-tasting protein thaumatin i. ''Acta Crystallogr Sect F Struct Biol Cryst Commun'', 67(Pt 6):652–8. |
*[Masuda et al., 2004] Masuda, T., Tamaki, S., Kaneko, R., Wada, R., Fujita, Y., Mehta, A., and Kitabatake, N. (2004). Cloning, expression and characterization of recombinant sweet-protein thaumatin ii using the methylotrophic yeast pichia pastoris. ''Biotechnol Bioeng'', 85(7):761–9. | *[Masuda et al., 2004] Masuda, T., Tamaki, S., Kaneko, R., Wada, R., Fujita, Y., Mehta, A., and Kitabatake, N. (2004). Cloning, expression and characterization of recombinant sweet-protein thaumatin ii using the methylotrophic yeast pichia pastoris. ''Biotechnol Bioeng'', 85(7):761–9. | ||
*[Moralejo et al., 1999] Moralejo, F. J., Cardoza, R. E., Gutierrez, S., and Martin, J. F. (1999). Thaumatin production in aspergillus awamori by use of expression cassettes with strong fungal promoters and high gene dosage. ''Appl Environ Microbiol'', 65(3):1168–74. | *[Moralejo et al., 1999] Moralejo, F. J., Cardoza, R. E., Gutierrez, S., and Martin, J. F. (1999). Thaumatin production in aspergillus awamori by use of expression cassettes with strong fungal promoters and high gene dosage. ''Appl Environ Microbiol'', 65(3):1168–74. | ||
Revision as of 23:12, 19 September 2012



Contents |
Thaumatin
We must admit, even we don't think that a "Münchner Hell" or a "Pils" would benefit from the distinct sweetness of Thaumatin, originally produced by Thaumacoccus daniellii.
So, why, o why have we chosen Thaumatin? There are a variety of arguments that have to be taken into consideration:
- Since experiments in the 1980ies, nobody tried to express Thaumatin via S. cerevisiae - so we saw the possibility to enhance the production and make it more efficient (as one of the biggest problems for industrial use of Thaumatin so far is the low yield efficiency) by codon usage optimization.
- If you think of the beers of the Anglo-Saxon world - stouts, ales - these are the sorts that could directly profit from the licorice-like sweetness, as they are generally more full-bodied and show a variety of caramel flavors.
- It opens the world of brewage to a whole new generation of lifestyle drinks - perhaps a bit too innovative for the conservative german beer market, but nevertheless there's quite a potential there for cutting-edge beverages with the soul of beer and the savor of all the fruits you can imagine. Thaumatin might serve as a low-carb ingredient to balance out the bitter and sour flavors of guava, grapefruits, currant - or even horseradish.
Background and principles
Thaumatin is a natural α+β-protein which is synthesized by the katamfe plant (Thaumatococcus daniellii) – a species of tropical flowering plants - and belongs to the thaumatin-like protein family. There exist different varieties of thaumatin, however, thaumatin I und thaumatin II are well characterized and differ only in one position (position 46 – without signaling sequence; thaumatin I Asn; thaumatin II Lys). Both are said to be 2000 to 100000 times sweeter than sucrose on molar basis, but the sweetness builds slow and lasts long.
Thaumatin is a single chain with 207 amino acids residues and eight disulfide bonds and a molecular weight of 22.2 kDa. It is highly water soluble, stable at heating (not for cooking, bakery, etc.) and stable under acidic conditions. The production of thaumatin is induced by an attack upon the plant by viroid pathogens. Thus it is involved in systematically acquired resistance and stress response.
Thaumatin has been approved as a sweetener in the European Union (E957).
The molecular and physiological effects of thaumatin
The sweet taste receptor is a heterodimeric receptor composed of T1R2 (also TAS1R2) and T1R3 (also TAS1R3) subunits. The large amino-terminal domains (NTD) of the T1R2 and T1R3 subunits have shown to be responsible for the primary ligand binding http://www.ncbi.nlm.nih.gov/pubmed?term=22450161 Maîtrepierre et al., 2012. In addition these receptors have a transmembrane heptahelical domain. T1R receptors belong to the family of class C G-Protein coupled receptors (GPCRs), which in this case means that through ligand binding an elevation of the cAMP concentration in the taste buds is induced [[http://www.ncbi.nlm.nih.gov/pubmed?term=19489607 Ide et al., 2009], [http://www.ncbi.nlm.nih.gov/pubmed?term=15087236 Ozeck et al., 2004]]. As a result a decrease in the intracellular cAMP accumulation is measured. Released calcium (Ca2+) seems to be another independent second messenger within the transduction of the taste response (downstream of taste receptors) http://www.ncbi.nlm.nih.gov/pubmed?term=16510847 Trubey et al., 2006.
However, not only sucralose or other sugars can bind with the NTDs of the sweet taste receptor, but also thaumatin http://www.ncbi.nlm.nih.gov/pubmed?term=19489607 Ide et al., 2009. It seems to have a longer lasting and stronger effect than sucralose.
Idea
The general idea is to create via genetic engineering of Saccharomyces cerevisiae a system that expresses thaumatin, respectively the direct precursor (preprothaumatin). The N-terminal "pre" part is the internal signalling sequence for secretion, the C-terminal "pro" sequence supports the correct and functional folding of thaumatin. Alternatively one could have used a fusion product of prothaumatin and the Mat-alalalalalpha-factor to achieve secretion.
Preferable seems to be the natural preprothaumatin, because of the expected higher yield http://www.ncbi.nlm.nih.gov/pubmed?term=21636903 Masuda et al., 2011 and the possibility that the pre-sequence is necessary for the correct procession http://www.ncbi.nlm.nih.gov/pubmed?term=17897626 Ide et al., 2007. A similar construct was used by the Kyoto University (Ide et al., submitted) in Pichia pastoris with a the pPIC6α expression vector with a high yield (especially with the preprothaumatin I gene and without the α-factor secretion signal).
To achieve the highest possible yield, we optimized the original gene sequence for best yeast codon usage (via GeneArt® GeneOptimizer®).
Remarks
Proof of principle
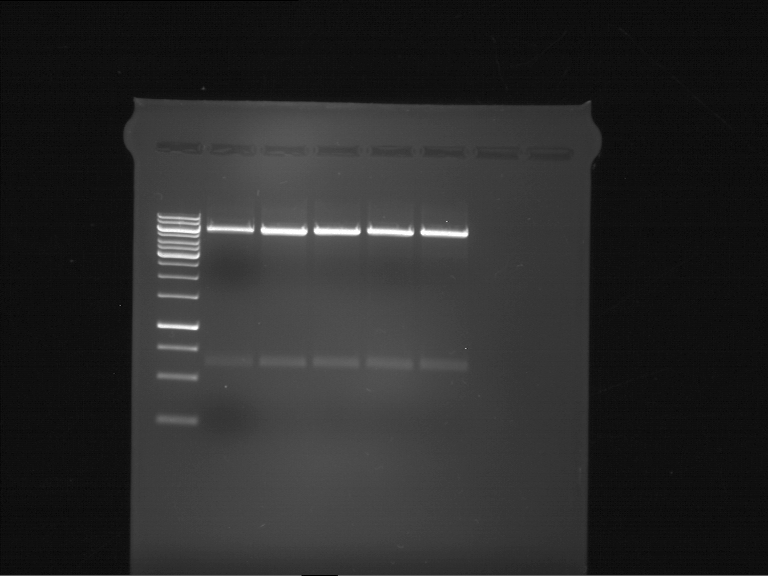
- First of all the existence of the preprothaumatin I / thaumatin I gene within the gene construct (via the Gal1-promotor in pYES2) needs to be proved (via control digest and sequencing)
- Thaumatin expression and secretion is detected via SDS-Page of the supernatant
- Purification: Ion exchange chromatography and mass spectrometry
Results
- Control digest pYes2_Preprothaumatin...:
- Annotated gene sequence, pYes2_Preprothaumatin.
- Picture of colonies.
- SDS-PAGE.
- IEX.
- MS.
References
- http://www.ncbi.nlm.nih.gov/pubmed?term=17897626 Ide et al., 2007 Ide, N., Masuda, T., and Kitabatake, N. (2007). Effects of pre- and pro-sequence of thaumatin on the secretion by Pichia pastoris. Biochem Biophys Res Commun, 363(3):708–14.
- http://www.ncbi.nlm.nih.gov/pubmed?term=19489607 Ide et al., 2009 Ide, N., Sato, E., Ohta, K., Masuda, T., and Kitabatake, N. (2009). Interactions of the sweet-tasting proteins thaumatin and lysozyme with the human sweet-taste receptor. J Agric Food Chem, 57(13):5884–90.
- [Lee et al., 1988] Lee, J. H., Weickmann, J. L., Koduri, R. K., Ghosh-Dastidar, P., Saito, K., Blair, L. C., Date, T., Lai, J. S., Hollenberg, S. M., and Kendall, R. L. (1988). Expression of synthetic thaumatin genes in yeast. Biochemistry, 27(14):5101–7.
- http://www.ncbi.nlm.nih.gov/pubmed?term=22450161 Maîtrepierre et al., 2012 Maîtrepierre, E., Sigoillot, M., Le Pessot, L., and Briand, L. (2012). Recombinant expression, in vitro refolding, and biophysical characterization of the n-terminal domain of t1r3 taste receptor. Protein Expr Purif, 83(1):75–83.
- http://www.ncbi.nlm.nih.gov/pubmed?term=21636903 Masuda et al., 2011 Masuda, T., Ohta, K., Mikami, B., and Kitabatake, N. (2011). High-resolution structure of the recombinant sweet-tasting protein thaumatin i. Acta Crystallogr Sect F Struct Biol Cryst Commun, 67(Pt 6):652–8.
- [Masuda et al., 2004] Masuda, T., Tamaki, S., Kaneko, R., Wada, R., Fujita, Y., Mehta, A., and Kitabatake, N. (2004). Cloning, expression and characterization of recombinant sweet-protein thaumatin ii using the methylotrophic yeast pichia pastoris. Biotechnol Bioeng, 85(7):761–9.
- [Moralejo et al., 1999] Moralejo, F. J., Cardoza, R. E., Gutierrez, S., and Martin, J. F. (1999). Thaumatin production in aspergillus awamori by use of expression cassettes with strong fungal promoters and high gene dosage. Appl Environ Microbiol, 65(3):1168–74.
- http://www.ncbi.nlm.nih.gov/pubmed?term=15087236 Ozeck et al., 2004 Ozeck, M., Brust, P., Xu, H., and Servant, G. (2004). Receptors for bitter, sweet and umami taste couple to inhibitory g protein signaling pathways. Eur J Pharmacol, 489(3):139–49.
- http://www.ncbi.nlm.nih.gov/pubmed?term=16510847 Trubey et al., 2006 Trubey, K. R., Culpepper, S., Maruyama, Y., Kinnamon, S. C., and Chaudhari, N. (2006). Tastants evoke camp signal in taste buds that is independent of calcium signaling. Am J Physiol Cell Physiol, 291(2):C237–44.
Others
- Masuda and Kitabatake (2006), Development of Biotechnological Production of Sweet Proteins http://www.jstage.jst.go.jp/article/jbb/102/5/375/_pdf
- Thaumatococcus daniellii mRNA forpreprothaumatin I, comeplete cds http://www.ncbi.nlm.nih.gov/nuccore/121945717?report=genbank
- Preprothaumatin I [Thaumatococcus daniellii], FASTA http://www.ncbi.nlm.nih.gov/protein/121945718?report=fasta
- Masuda, Ohta, Mikami, Kitabatake (2011), High-resolution structure of the recombinant sweet-tasting protein thaumatin I http://repository.kulib.kyoto-u.ac.jp/dspace/bitstream/2433/142313/1/S174430911101373X.pdf
- Nobuyuki Ide, Tetsuya Masuda, Naofumi Kitabatake (2007), Effects of pre- and pro-sequence of thaumatin on the secretion by Pichia pastoris http://www.sciencedirect.com/science/article/pii/S0006291X07019900
- David P. Clark, Nanette J. Pazdernik (2009); “Molekulare Biotechnologie”: 306 – 309
- yeastgenome.org http://www.yeastgenome.org/
 "
"