Team:METU/KillSwitchOverview
From 2012.igem.org
Dumankolik (Talk | contribs) |
Dumankolik (Talk | contribs) |
||
| (29 intermediate revisions not shown) | |||
| Line 35: | Line 35: | ||
</head> | </head> | ||
| - | <body> | + | <body style="background-image: url(https://static.igem.org/mediawiki/2012/c/cb/METU-team-logo-leaves.png); background-repeat: no-repeat; background-attachment: fixed; background-position:center;"> |
| + | |||
| + | |||
<!-- Header --> | <!-- Header --> | ||
<div id="header"> | <div id="header"> | ||
| Line 50: | Line 52: | ||
<li><a href="https://2012.igem.org/Team:METU/Team"><span>Team</span></a></li> | <li><a href="https://2012.igem.org/Team:METU/Team"><span>Team</span></a></li> | ||
| - | <li><a href=" | + | <li><a href="#"><span>Project</span></a> |
<div class="dd-holder"> | <div class="dd-holder"> | ||
<div class="dd-t"></div> | <div class="dd-t"></div> | ||
<div class="dd"> | <div class="dd"> | ||
<ul> | <ul> | ||
| - | <li><a href="https://2012.igem.org/Team:METU/ | + | <li><a href="https://2012.igem.org/Team:METU/ProjectOverview"><span>Overview</span></a></li> |
| - | <li><a href="https://2012.igem.org/Team:METU/CODH"><span> | + | <li><a href="https://2012.igem.org/Team:METU/CODH"><span>CO Converter</span></a></li> |
<li><a href="https://2012.igem.org/Team:METU/KillSwitchOverview"><span>Kill-Switch</span></a></li> | <li><a href="https://2012.igem.org/Team:METU/KillSwitchOverview"><span>Kill-Switch</span></a></li> | ||
<li><a href="https://2012.igem.org/Team:METU/CellLimiter"><span>Cell Limiter</span></a></li> | <li><a href="https://2012.igem.org/Team:METU/CellLimiter"><span>Cell Limiter</span></a></li> | ||
| Line 70: | Line 72: | ||
<li><a href="https://2012.igem.org/Team:METU/Results"><span>Results</span></a></li> | <li><a href="https://2012.igem.org/Team:METU/Results"><span>Results</span></a></li> | ||
| - | <li><a href=" | + | <li><a href="#"><span>Notebook</span></a> |
<div class="dd-holder"> | <div class="dd-holder"> | ||
<div class="dd-t"></div> | <div class="dd-t"></div> | ||
<div class="dd"> | <div class="dd"> | ||
<ul> | <ul> | ||
| - | + | ||
<li><a href="/Team:METU/Timeline">Timeline</a></li> | <li><a href="/Team:METU/Timeline">Timeline</a></li> | ||
| - | <li><a href="/Team:METU/Brainstorm"> | + | <li><a href="/Team:METU/Brainstorm">Brainstorming</a></li> |
<li><a href="https://2012.igem.org/Team:METU/Protocols">Protocols</a></li> | <li><a href="https://2012.igem.org/Team:METU/Protocols">Protocols</a></li> | ||
| - | + | ||
<li class="last"><a href="/Team:METU/Judging">Judging</a></li> | <li class="last"><a href="/Team:METU/Judging">Judging</a></li> | ||
</ul> | </ul> | ||
| Line 131: | Line 133: | ||
</div> | </div> | ||
<!-- End Header --> | <!-- End Header --> | ||
| - | <!-- Slider --> | + | <!-- Slider --> |
| - | <div id="slider2"> | + | <div id="slider2"> |
| - | + | <div class="shell"> | |
| - | + | <div class="slider-holder2"> | |
| - | + | <h1>Kill Switch</h1> | |
| - | + | <div class="cl"> </div> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | <!-- End Slider --> | ||
<!-- Main --> | <!-- Main --> | ||
| - | + | <div class="shell"> | |
| - | + | <div class="col2"> | |
| - | + | <table id="toc" class="toc" style=" | |
| - | + | width: 400px; | |
| - | + | "> | |
| - | + | <tbody><tr> | |
| - | + | ||
| - | + | ||
| - | <tbody><tr> | + | |
<td> | <td> | ||
<div id="table"> | <div id="table"> | ||
| - | <h2><b> | + | <h2><b> Kill Switch </b></h2> |
| - | <span class="toctoggle"> | + | <span class="toctoggle"> </span></div> |
<ul> | <ul> | ||
| - | <li class="toclevel-1 tocsection-1"><a href="# | + | <li class="toclevel-1 tocsection-1"><a href="#pcr"><span class="tocnumber">1.</span> <span class="toctext">Overview</span></a></li> |
| - | <li class="toclevel- | + | <li class="toclevel-1 tocsection-2"><a href="#colony"><span class="tocnumber">2.</span> <span class="toctext">How the System Works?</span></a></li> |
| - | <li class="toclevel- | + | <li class="toclevel-1 tocsection-3"><a href="#pcr1"><span class="tocnumber">3.</span> <span class="toctext">Modelling</span></a></li> |
| + | <li class="toclevel-1 tocsection-4"><a href="#cultures"><span class="tocnumber">4.</span> <span class="toctext">References</span></a> | ||
</ul> | </ul> | ||
| - | |||
</td> | </td> | ||
</tr> | </tr> | ||
</tbody></table> | </tbody></table> | ||
| - | </div> | + | </div> |
| + | </div> | ||
| + | |||
| + | <div id="main"> | ||
| + | <div class="shell"> | ||
<div class="col2"> | <div class="col2"> | ||
| - | <div id=" | + | <div id="Overview"> |
| - | <h2>Overview</h2> | + | |
| + | <h2 id="pcr">Overview</h2> | ||
<span> | <span> | ||
<p> | <p> | ||
| - | + | Kill switch is a safety system which is necessary to control the release of genetically modified | |
| - | + | organisms (GMOs) into the environment. Since we aimed to construct a biofilm with GMOs which will convert CO to CO2, we needed a kill switch that can prevent our system to cause any harm to the environment. In order to achieve that aim we used the kill switch designed by Berkeley 2008 iGEM Team (Bba_K112808) and also we tried to develop it. | |
| - | + | ||
| - | + | ||
| - | + | ||
| Line 227: | Line 186: | ||
</span> | </span> | ||
| - | <h2 id=" | + | <h2 id="colony" >How The System Works ?</h2> |
<p> | <p> | ||
| - | + | ||
| - | When | + | In order to construct our kill switch system we needed a reagent that E.Coli will grow and in the absence of that reagent all the bacteria should die. For that purpose we prefered to use IPTG (Isopropyl-beta-D-thiogalactopyranoside) because it cannot be metabolised by E.Coli and that is why its concentration remains constant in the cell.<b>[1]</b> |
| + | |||
| + | When IPTG is present in the cell it will bind to LacI which can be produced by the promoter J23116. Basal LacI expression is already present in E.Coli but at low levels and it is hard to control the system with that much LacI. Hence, we prefered to produce it with the help of J23116. | ||
| + | |||
| + | Our kill switch system includes the expression of endolysin, holin, antiholin and their production is controlled with the initial concentration of IPTG in the environment. Actually | ||
| + | cell lysis can occur without IPTG but the lysis rate will be high. By using IPTG we aimed to keep our system under control. | ||
| + | |||
| + | <p> <b>Endolysin-Holin System;</b> Endolysin and holin are the proteins needed for the cell lysis. Endolysin is a lysozyme and its funtion is to hydrolyze the peptidoglycan bacterial cell wall but it needs holin to form pores on inner membrane, then it can reach periplasm and cell lysis occur. | ||
| + | |||
</p> | </p> | ||
| - | + | </p> | |
| - | + | ||
| + | <p> | ||
| + | <b> The fate of the Gram-negative envelope during holin-endolysin lysis:[2]</b> | ||
| + | |||
| + | <img src="https://static.igem.org/mediawiki/2012/9/97/Metu-photos-model27.png.jpg" alt="From the journal Trends in Microbiology"> | ||
| + | </p> | ||
| + | <p> | ||
| + | In this figure holins are shown as clear ovals and endolysin is shown as notched circles. | ||
| + | </p> | ||
| + | <ul> | ||
| + | <li><b>a-)Pre-Hole Configuration:</b> Accumulation of holin is present in the inner membrane, while endolysin accumulates in the cytosol. Holin action can be prevented with the inhibitors (shown with star and black ovals).</li> | ||
| + | <li> <b>b-) Hole Configuration:</b> When the holes are formed by holin in the inner membrane, endolysin can attack the murein which is important for cell lysis to occur.</li> | ||
| + | </ul> | ||
| + | | ||
| + | |||
| + | |||
| + | <p><b>Antiholin;</b> It is the protein that blocks the function of holin and it is important for the regulation of cell lysis.<b>[3]</b> | ||
| + | |||
| + | </p> | ||
| + | <p id="Overview"> | ||
| + | In our system antiholin is constitutively produced by the promoter J23116 and when antiholin level is in between 1000-3000 molecules, the cell will die.<b>[4]</b> Actually,it is the number of free holin molecules that determines if the cell will die or not. | ||
| + | </p> | ||
</div> | </div> | ||
| - | <div id=" | + | <div id="pcr1"> |
| - | <h2 | + | <h2>Modelling</h2> |
<span> | <span> | ||
<p> | <p> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | < | + | <div id="main"> |
| - | + | <h2><b> KILL-SWITCH MODEL </b></h2> | |
| - | + | ||
| - | + | ||
| - | < | + | |
<span> | <span> | ||
| | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| + | The aim of kill-switch model is to predict the behavior of kill-switch mechanism in different IPTG concentrations. We first tried to model mechanism by the help of ODEs. Then we used SimBiology toolbox of MATLAB to simulate system. | ||
<p> | <p> | ||
<b>Introduction:</b> | <b>Introduction:</b> | ||
</p> | </p> | ||
| - | |||
<p> | <p> | ||
| - | + | As can be seen from the diagram our circuit involves constitutive production of LacI. LacI Promoter is negatively regulated by LacI. The control mechanism of our system is inhibition of LacI by IPTG molecule. Since LacI is inhibited by IPTG its effect on LacI promoter changes with respect to IPTG concentration. | |
| - | + | Holin and Endolysin expression is controlled by cI promoter which is negatively regulated by cI. | |
| - | + | Our first assumption in the model is that when the free Holin molecules, which means number of Holin molecules minus number of Antiholin molecules, reach 3000[ref] cell lysis occurs. | |
| - | + | Because cI concentration depends on the activity of LacI promoter, we can conclude that in the presence of IPTG the concentration of cI will be high and expression of Holin and Endolysin will be lowered. Due to the constitutive production of Antiholin, we can say that free Holin molecules will be lowered and chance of cell lysis to occur will drop. However, to understand the overall behaviour of system with respect to IPTG concentration we need to model and simulate the system. | |
| + | <img src="https://static.igem.org/mediawiki/2012/2/24/Metu-photos-model1.png" alt="" " | ||
</p> | </p> | ||
| Line 284: | Line 257: | ||
In our model, we included all processes shown below. | In our model, we included all processes shown below. | ||
| - | <img src="https://static.igem.org/mediawiki/2012/8/ | + | <img src="https://static.igem.org/mediawiki/2012/8/82/Metu-photos-model2.jpg" alt="" "> |
<p><b>Equations for mRNA transcription</b></p> | <p><b>Equations for mRNA transcription</b></p> | ||
| Line 291: | Line 264: | ||
| | ||
| - | <p><img src="https://static.igem.org/mediawiki/2012/ | + | <p><img src="https://static.igem.org/mediawiki/2012/2/29/Metu-photos-model3.png" alt="" "> |
</p> | </p> | ||
| | ||
| - | <p><img src="https://static.igem.org/mediawiki/2012/8/ | + | <p><img src="https://static.igem.org/mediawiki/2012/8/81/Metu-photos-model4.png" alt="" "> |
</p> | </p> | ||
| | ||
| - | <p><img src="https://static.igem.org/mediawiki/2012/ | + | <p><img src="https://static.igem.org/mediawiki/2012/c/cd/Metu-photos-model5.png" alt="" "> |
</p> | </p> | ||
| | ||
| - | <p><img src="https://static.igem.org/mediawiki/2012/ | + | <p><img src="https://static.igem.org/mediawiki/2012/9/9f/Metu-photos-model6.png" alt="" "> |
</p> | </p> | ||
| | ||
| - | + | <p><img src="https://static.igem.org/mediawiki/2012/8/8a/Metu-photos-model7.png" alt="" "> | |
| - | < | + | </p> |
| - | + | | |
| - | + | <b> Equations for Protein Translation </b> | |
| | ||
| | ||
| | ||
| - | <p><img src="https://static.igem.org/mediawiki/2012/ | + | <p><img src="https://static.igem.org/mediawiki/2012/9/9c/Metu-photos-model8.png" alt="" /> |
</p> | </p> | ||
| | ||
| - | <p><img src="https://static.igem.org/mediawiki/2012/ | + | <p><img src="https://static.igem.org/mediawiki/2012/4/45/Metu-photos-model9.png" alt="" /> |
</p> | </p> | ||
| | ||
| - | <p><img src="https://static.igem.org/mediawiki/2012/ | + | <p><img src="https://static.igem.org/mediawiki/2012/1/12/Metu-photos-model10.png" alt="" /> |
</p> | </p> | ||
| | ||
| - | <p><img src="https://static.igem.org/mediawiki/2012/ | + | <p><img src="https://static.igem.org/mediawiki/2012/c/c6/Metu-photos-model11.png" alt="" /> |
</p> | </p> | ||
| | ||
| - | <p><img src="https://static.igem.org/mediawiki/2012/ | + | <p><img src="https://static.igem.org/mediawiki/2012/5/5b/Metu-photos-model12.png" alt="" /> |
</p> | </p> | ||
| | ||
| - | <p><img src="https://static.igem.org/mediawiki/2012/ | + | <p><img src="https://static.igem.org/mediawiki/2012/a/ab/Metu-photos-model13.png" alt="" /> |
</p> | </p> | ||
| | ||
| - | <p><img src="https://static.igem.org/mediawiki/2012/ | + | <p><img src="https://static.igem.org/mediawiki/2012/e/e7/Metu-photos-model14.png" alt="" /> |
</p> | </p> | ||
| | ||
| - | |||
| - | |||
<table class="ListTable31" border="1" cellspacing="0" cellpadding="0" style="border-collapse: | <table class="ListTable31" border="1" cellspacing="0" cellpadding="0" style="border-collapse: | ||
collapse;border:none;mso-border-alt:solid black .5pt;mso-border-themecolor: | collapse;border:none;mso-border-alt:solid black .5pt;mso-border-themecolor: | ||
| Line 343: | Line 314: | ||
mso-border-left-themecolor:text1;background:black;mso-background-themecolor: | mso-border-left-themecolor:text1;background:black;mso-background-themecolor: | ||
text1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | text1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal;mso-yfti-cnfc:517"><b><span | + | normal;mso-yfti-cnfc:517"><b><span style="font-size:14.0pt;mso-bidi-font-size: |
| - | + | 11.0pt;color:white;mso-themecolor:background1;mso-ansi-language:EN-US">Parameter<o:p></o:p></span></b></p> | |
| - | + | ||
</td> | </td> | ||
<td valign="top" style="border-top:solid black 1.0pt;mso-border-top-themecolor: | <td valign="top" style="border-top:solid black 1.0pt;mso-border-top-themecolor: | ||
| Line 354: | Line 324: | ||
mso-border-right-themecolor:text1;background:black;mso-background-themecolor: | mso-border-right-themecolor:text1;background:black;mso-background-themecolor: | ||
text1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | text1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal;mso-yfti-cnfc:1"><b><span | + | normal;mso-yfti-cnfc:1"><b><span style="font-size:14.0pt;mso-bidi-font-size: |
| - | + | 11.0pt;color:white;mso-themecolor:background1;mso-ansi-language:EN-US">Description<o:p></o:p></span></b></p> | |
| - | + | ||
</td> | </td> | ||
</tr> | </tr> | ||
| Line 366: | Line 335: | ||
mso-border-bottom-alt:solid black .5pt;mso-border-bottom-themecolor:text1; | mso-border-bottom-alt:solid black .5pt;mso-border-bottom-themecolor:text1; | ||
background:white;mso-background-themecolor:background1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | background:white;mso-background-themecolor:background1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal;mso-yfti-cnfc:68"><span | + | normal;mso-yfti-cnfc:68"><span style="font-size:14.0pt;mso-bidi-font-size: |
11.0pt;mso-ansi-language:EN-US;mso-bidi-font-weight:bold">N<o:p></o:p></span></p> | 11.0pt;mso-ansi-language:EN-US;mso-bidi-font-weight:bold">N<o:p></o:p></span></p> | ||
</td> | </td> | ||
| Line 375: | Line 344: | ||
text1;mso-border-right-alt:solid black .5pt;mso-border-right-themecolor:text1; | text1;mso-border-right-alt:solid black .5pt;mso-border-right-themecolor:text1; | ||
padding:1.45pt 5.4pt 1.45pt 5.4pt"> | padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal;mso-yfti-cnfc:64"><span | + | normal;mso-yfti-cnfc:64"><span style="font-size:14.0pt;mso-bidi-font-size: |
11.0pt;mso-ansi-language:EN-US">Plasmid copy number<o:p></o:p></span></p> | 11.0pt;mso-ansi-language:EN-US">Plasmid copy number<o:p></o:p></span></p> | ||
</td> | </td> | ||
| Line 384: | Line 353: | ||
text1;mso-border-left-alt:solid black .5pt;mso-border-left-themecolor:text1; | text1;mso-border-left-alt:solid black .5pt;mso-border-left-themecolor:text1; | ||
background:white;mso-background-themecolor:background1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | background:white;mso-background-themecolor:background1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal;mso-yfti-cnfc:4"><span class="SpellE"><span | + | normal;mso-yfti-cnfc:4"><span class="SpellE"><span style="font-size:14.0pt; |
| - | + | mso-bidi-font-size:11.0pt;mso-ansi-language:EN-US;mso-bidi-font-weight:bold">P<sub>x</sub></span></span><sub><span style="font-size:14.0pt;mso-bidi-font-size:11.0pt;mso-ansi-language:EN-US; | |
| - | + | mso-bidi-font-weight:bold"><o:p></o:p></span></sub></p> | |
| - | + | ||
</td> | </td> | ||
<td valign="top" style="border:none;border-right:solid black 1.0pt;mso-border-right-themecolor: | <td valign="top" style="border:none;border-right:solid black 1.0pt;mso-border-right-themecolor: | ||
text1;mso-border-right-alt:solid black .5pt;mso-border-right-themecolor:text1; | text1;mso-border-right-alt:solid black .5pt;mso-border-right-themecolor:text1; | ||
padding:1.45pt 5.4pt 1.45pt 5.4pt"> | padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal"><span | + | normal"><span style="font-size:14.0pt;mso-bidi-font-size:11.0pt;mso-ansi-language: |
| - | + | EN-US">Promoter Strength<o:p></o:p></span></p> | |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 404: | Line 372: | ||
mso-border-bottom-alt:solid black .5pt;mso-border-bottom-themecolor:text1; | mso-border-bottom-alt:solid black .5pt;mso-border-bottom-themecolor:text1; | ||
background:white;mso-background-themecolor:background1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | background:white;mso-background-themecolor:background1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal;mso-yfti-cnfc:68"><span | + | normal;mso-yfti-cnfc:68"><span style="font-size:14.0pt;mso-bidi-font-size: |
11.0pt;mso-bidi-font-family:Calibri;mso-bidi-theme-font:minor-latin; | 11.0pt;mso-bidi-font-family:Calibri;mso-bidi-theme-font:minor-latin; | ||
| - | mso-ansi-language:EN-US;mso-bidi-font-weight:bold"> | + | mso-ansi-language:EN-US;mso-bidi-font-weight:bold">a</span><sub><span style="font-size:14.0pt;mso-bidi-font-size:11.0pt;mso-ansi-language:EN-US; |
| - | + | mso-bidi-font-weight:bold">x<o:p></o:p></span></sub></p> | |
</td> | </td> | ||
<td valign="top" style="border:solid black 1.0pt;mso-border-themecolor:text1; | <td valign="top" style="border:solid black 1.0pt;mso-border-themecolor:text1; | ||
| Line 415: | Line 383: | ||
text1;mso-border-right-alt:solid black .5pt;mso-border-right-themecolor:text1; | text1;mso-border-right-alt:solid black .5pt;mso-border-right-themecolor:text1; | ||
padding:1.45pt 5.4pt 1.45pt 5.4pt"> | padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal;mso-yfti-cnfc:64"><span | + | normal;mso-yfti-cnfc:64"><span style="font-size:14.0pt;mso-bidi-font-size: |
11.0pt;mso-ansi-language:EN-US">Degradation Rate<o:p></o:p></span></p> | 11.0pt;mso-ansi-language:EN-US">Degradation Rate<o:p></o:p></span></p> | ||
</td> | </td> | ||
| Line 424: | Line 392: | ||
text1;mso-border-left-alt:solid black .5pt;mso-border-left-themecolor:text1; | text1;mso-border-left-alt:solid black .5pt;mso-border-left-themecolor:text1; | ||
background:white;mso-background-themecolor:background1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | background:white;mso-background-themecolor:background1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal;mso-yfti-cnfc:4"><span class="SpellE"><span | + | normal;mso-yfti-cnfc:4"><span class="SpellE"><span style="font-size:14.0pt; |
| - | + | mso-bidi-font-size:11.0pt;mso-ansi-language:EN-US;mso-bidi-font-weight:bold">K<sub>x</sub></span></span><sub><span style="font-size:14.0pt;mso-bidi-font-size:11.0pt;mso-ansi-language:EN-US; | |
| - | + | mso-bidi-font-weight:bold"><o:p></o:p></span></sub></p> | |
| - | + | ||
</td> | </td> | ||
<td valign="top" style="border:none;border-right:solid black 1.0pt;mso-border-right-themecolor: | <td valign="top" style="border:none;border-right:solid black 1.0pt;mso-border-right-themecolor: | ||
text1;mso-border-right-alt:solid black .5pt;mso-border-right-themecolor:text1; | text1;mso-border-right-alt:solid black .5pt;mso-border-right-themecolor:text1; | ||
padding:1.45pt 5.4pt 1.45pt 5.4pt"> | padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal"><span | + | normal"><span style="font-size:14.0pt;mso-bidi-font-size:11.0pt;mso-ansi-language: |
| - | + | EN-US">Disassociation (Equilibrium) Constant<o:p></o:p></span></p> | |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 444: | Line 411: | ||
mso-border-bottom-alt:solid black .5pt;mso-border-bottom-themecolor:text1; | mso-border-bottom-alt:solid black .5pt;mso-border-bottom-themecolor:text1; | ||
background:white;mso-background-themecolor:background1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | background:white;mso-background-themecolor:background1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal;mso-yfti-cnfc:68"><span class="SpellE"><span | + | normal;mso-yfti-cnfc:68"><span class="SpellE"><span style="font-size:14.0pt; |
| - | + | mso-bidi-font-size:11.0pt;mso-ansi-language:EN-US;mso-bidi-font-weight:bold">n<sub>x</sub></span></span><sub><span style="font-size:14.0pt;mso-bidi-font-size:11.0pt;mso-ansi-language:EN-US; | |
mso-bidi-font-weight:bold"><o:p></o:p></span></sub></p> | mso-bidi-font-weight:bold"><o:p></o:p></span></sub></p> | ||
</td> | </td> | ||
| Line 454: | Line 421: | ||
text1;mso-border-right-alt:solid black .5pt;mso-border-right-themecolor:text1; | text1;mso-border-right-alt:solid black .5pt;mso-border-right-themecolor:text1; | ||
padding:1.45pt 5.4pt 1.45pt 5.4pt"> | padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal;mso-yfti-cnfc:64"><span | + | normal;mso-yfti-cnfc:64"><span style="font-size:14.0pt;mso-bidi-font-size: |
11.0pt;mso-ansi-language:EN-US">Hill Coefficient<o:p></o:p></span></p> | 11.0pt;mso-ansi-language:EN-US">Hill Coefficient<o:p></o:p></span></p> | ||
</td> | </td> | ||
| Line 463: | Line 430: | ||
text1;mso-border-left-alt:solid black .5pt;mso-border-left-themecolor:text1; | text1;mso-border-left-alt:solid black .5pt;mso-border-left-themecolor:text1; | ||
background:white;mso-background-themecolor:background1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | background:white;mso-background-themecolor:background1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal;mso-yfti-cnfc:4"><span class="SpellE"><span | + | normal;mso-yfti-cnfc:4"><span class="SpellE"><span style="font-size:14.0pt; |
| - | + | mso-bidi-font-size:11.0pt;mso-ansi-language:EN-US;mso-bidi-font-weight:bold">t<sub>x</sub></span></span><sub><span style="font-size:14.0pt;mso-bidi-font-size:11.0pt;mso-ansi-language:EN-US; | |
| - | + | mso-bidi-font-weight:bold"><o:p></o:p></span></sub></p> | |
| - | + | ||
</td> | </td> | ||
<td valign="top" style="border:none;border-right:solid black 1.0pt;mso-border-right-themecolor: | <td valign="top" style="border:none;border-right:solid black 1.0pt;mso-border-right-themecolor: | ||
text1;mso-border-right-alt:solid black .5pt;mso-border-right-themecolor:text1; | text1;mso-border-right-alt:solid black .5pt;mso-border-right-themecolor:text1; | ||
padding:1.45pt 5.4pt 1.45pt 5.4pt"> | padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal"><span | + | normal"><span style="font-size:14.0pt;mso-bidi-font-size:11.0pt;mso-ansi-language: |
| - | + | EN-US">Translation Rate<o:p></o:p></span></p> | |
</td> | </td> | ||
</tr> | </tr> | ||
| Line 483: | Line 449: | ||
mso-border-bottom-alt:solid black .5pt;mso-border-bottom-themecolor:text1; | mso-border-bottom-alt:solid black .5pt;mso-border-bottom-themecolor:text1; | ||
background:white;mso-background-themecolor:background1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | background:white;mso-background-themecolor:background1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal;mso-yfti-cnfc:68"><span class="SpellE"><span | + | normal;mso-yfti-cnfc:68"><span class="SpellE"><span style="font-size:14.0pt; |
| - | + | mso-bidi-font-size:11.0pt;mso-ansi-language:EN-US;mso-bidi-font-weight:bold">k<sub>x</sub></span></span><sub><span style="font-size:14.0pt;mso-bidi-font-size:11.0pt;mso-ansi-language:EN-US; | |
mso-bidi-font-weight:bold"><o:p></o:p></span></sub></p> | mso-bidi-font-weight:bold"><o:p></o:p></span></sub></p> | ||
</td> | </td> | ||
| Line 493: | Line 459: | ||
text1;mso-border-right-alt:solid black .5pt;mso-border-right-themecolor:text1; | text1;mso-border-right-alt:solid black .5pt;mso-border-right-themecolor:text1; | ||
padding:1.45pt 5.4pt 1.45pt 5.4pt"> | padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal;mso-yfti-cnfc:64"><span | + | normal;mso-yfti-cnfc:64"><span style="font-size:14.0pt;mso-bidi-font-size: |
11.0pt;mso-ansi-language:EN-US">Binding on rate<o:p></o:p></span></p> | 11.0pt;mso-ansi-language:EN-US">Binding on rate<o:p></o:p></span></p> | ||
</td> | </td> | ||
| Line 502: | Line 468: | ||
text1;mso-border-left-alt:solid black .5pt;mso-border-left-themecolor:text1; | text1;mso-border-left-alt:solid black .5pt;mso-border-left-themecolor:text1; | ||
background:white;mso-background-themecolor:background1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | background:white;mso-background-themecolor:background1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal;mso-yfti-cnfc:4"><span | + | normal;mso-yfti-cnfc:4"><span style="font-size:14.0pt;mso-bidi-font-size: |
11.0pt;mso-ansi-language:EN-US;mso-bidi-font-weight:bold">k<sub>-x<o:p></o:p></sub></span></p> | 11.0pt;mso-ansi-language:EN-US;mso-bidi-font-weight:bold">k<sub>-x<o:p></o:p></sub></span></p> | ||
</td> | </td> | ||
| Line 509: | Line 475: | ||
text1;mso-border-right-alt:solid black .5pt;mso-border-right-themecolor:text1; | text1;mso-border-right-alt:solid black .5pt;mso-border-right-themecolor:text1; | ||
padding:1.45pt 5.4pt 1.45pt 5.4pt"> | padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal"><span | + | normal"><span style="font-size:14.0pt;mso-bidi-font-size:11.0pt;mso-ansi-language: |
| - | + | EN-US">Binding off rate<o:p></o:p></span></p> | |
</td> | </td> | ||
</tr> | </tr> | ||
| - | <tr style="mso-yfti-irow:8"> | + | <tr style="mso-yfti-irow:8;mso-yfti-lastrow:yes"> |
<td valign="top" style="border:solid black 1.0pt;mso-border-themecolor:text1; | <td valign="top" style="border:solid black 1.0pt;mso-border-themecolor:text1; | ||
border-right:none;mso-border-top-alt:solid black .5pt;mso-border-top-themecolor: | border-right:none;mso-border-top-alt:solid black .5pt;mso-border-top-themecolor: | ||
| Line 520: | Line 486: | ||
mso-border-bottom-alt:solid black .5pt;mso-border-bottom-themecolor:text1; | mso-border-bottom-alt:solid black .5pt;mso-border-bottom-themecolor:text1; | ||
background:white;mso-background-themecolor:background1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | background:white;mso-background-themecolor:background1;padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal;mso-yfti-cnfc:68"><span class="SpellE"><span | + | normal;mso-yfti-cnfc:68"><span class="SpellE"><span style="font-size:14.0pt; |
| - | + | mso-bidi-font-size:11.0pt;mso-ansi-language:EN-US;mso-bidi-font-weight:bold">D<sub>x</sub></span></span><sub><span style="font-size:14.0pt;mso-bidi-font-size:11.0pt;mso-ansi-language:EN-US; | |
mso-bidi-font-weight:bold"><o:p></o:p></span></sub></p> | mso-bidi-font-weight:bold"><o:p></o:p></span></sub></p> | ||
</td> | </td> | ||
| Line 530: | Line 496: | ||
text1;mso-border-right-alt:solid black .5pt;mso-border-right-themecolor:text1; | text1;mso-border-right-alt:solid black .5pt;mso-border-right-themecolor:text1; | ||
padding:1.45pt 5.4pt 1.45pt 5.4pt"> | padding:1.45pt 5.4pt 1.45pt 5.4pt"> | ||
| - | <p class="MsoNormal" style="margin-bottom: | + | <p class="MsoNormal" style="margin-bottom:0in;margin-bottom:.0001pt;line-height: |
| - | normal;mso-yfti-cnfc:64"><span | + | normal;mso-yfti-cnfc:64"><span style="font-size:14.0pt;mso-bidi-font-size: |
11.0pt;mso-ansi-language:EN-US">Diffusion rate<o:p></o:p></span></p> | 11.0pt;mso-ansi-language:EN-US">Diffusion rate<o:p></o:p></span></p> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
</td> | </td> | ||
</tr> | </tr> | ||
</tbody></table> | </tbody></table> | ||
| - | |||
</span> | </span> | ||
| + | &nsbp; | ||
| + | &nsbp; | ||
| + | <p> <b> Simulation Results </b> </p> | ||
| + | When the IPTG concentration is 0; | ||
| + | <p><img src="https://static.igem.org/mediawiki/2012/c/c3/Metu-photos-model15.png.jpg" alt="" /></p> | ||
| + | <p>Individual graphs can be accessed <a href="https://2012.igem.org/Team:METU/KillSwitchOverview/1"><span>here</span></a>. | ||
| + | |||
| + | |||
| + | |||
| + | Because the critical point in kill-switch is “Holin-Antiholin” molecules, we ran simulation in different IPTG concentrations and to understand result easier we select the outputs as Holin and Antiholin. Since translation rate of endolysin is more than Holin and Antiholin we can assume that there will be enough endolysin to make cell lysis occur. Also from the graph above we can understand that endolysin will be in sufficient amount. | ||
| + | </p> | ||
| + | <p><img src="https://static.igem.org/mediawiki/2012/b/bf/Metu-photos-model16.png.jpg" alt="" height="371px" width="727px"/></p> | ||
| + | |||
| + | <p><img src="https://static.igem.org/mediawiki/2012/b/ba/Metu-photos-model17.png.jpg" alt="" height="371px" width="727px"/></p> | ||
| + | |||
| + | <p><img src="https://static.igem.org/mediawiki/2012/0/06/Metu-photos-model18.png.jpg" alt="" height="371px" width="727px" /></p> | ||
| + | |||
| + | <p><img src="https://static.igem.org/mediawiki/2012/2/2d/Metu-photos-model19.png.jpg" alt="" height="371px" width="727px"/></p> | ||
| + | |||
| + | |||
| + | |||
| + | <p>By the help of our model, we can conclude that when there is no IPTG molecule, cell lysis occur in 275 s. However when there is 100000 IPTG molecules, cell needs 2290 s to lyse. As can be seen from graphs until a very high IPTG concentration like in the Run14, the increase in Holin can eventually cause lysis. As a result of our model we concluded that after an IPTG concentration of 700000 molecule cell lysis will not occur. We are not considering the generation time of cell in this model since it can later be used for different organisms. If we consider e.coli with a generation time of 20 min, even in the medium with IPTG of 100000 molecules, it will divide before it can lyse. | ||
| + | </p> | ||
| + | |||
| + | <p>When IPTG concentration is 700000;</p> | ||
| + | <p><img src="https://static.igem.org/mediawiki/2012/3/34/Metu-photos-model20.png.jpg" alt="" height="371px" width="727px" /></p> | ||
| + | <p>Individual graphs can be accessed <a href="https://2012.igem.org/Team:METU/KillSwitchOverview/2"><span>here</span></a>.</p> | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <p><b> Sensivity Analysis </b></p> | ||
| + | <p>Since the challenging part of modelling is choice of parameters, we conducted a sensitivity analysis to see how much result of our model depends on parameters. By the help of those, the critical values to be measured through experiments can be understood easily. | ||
| + | </p> | ||
| + | <p><img src="https://static.igem.org/mediawiki/2012/d/d1/Metu-photos-model21.png.jpg" alt="" height="371px" width="727px" /></p> | ||
| + | <p><img src="https://static.igem.org/mediawiki/2012/8/85/Metu-photos-model22.png.jpg" alt="" height="371px" width="727px" /></p> | ||
| + | <p><img src="https://static.igem.org/mediawiki/2012/8/81/Metu-photos-model23.png.jpg" alt="" height="371px" width="727px"/></p> | ||
| + | <p><img src="https://static.igem.org/mediawiki/2012/4/44/Metu-photos-model24.png.jpg" alt="" height="371px" width="727px"/></p> | ||
| + | <p><img src="https://static.igem.org/mediawiki/2012/4/43/Metu-photos-model25.png.jpg" alt="" height="371px" width="727px"/></p> | ||
| + | <p><img class="bigImg" src="https://static.igem.org/mediawiki/2012/5/5c/Metu-photos-model26.png.jpg" alt=""height="371px" width="727px" onclick="live()"/> | ||
| + | <img class="bigImg" style="visibility:hidden" src="https://static.igem.org/mediawiki/2012/5/5c/Metu-photos-model26.png.jpg" /> | ||
| + | </p> | ||
| + | |||
| + | |||
| + | </div> | ||
| + | |||
| + | <h2 id="cultures"><b> References </b><h2/> | ||
| + | <div id ="ref"> | ||
| + | <div class="col2"> | ||
| + | <FONT SIZE=2> | ||
| + | <p><b>[1]</b>Hansen LH, Knudsen S., Sorensen SJ. (1998). The effect of the lacY gene on the induction of IPTG inducible promoters, studied in Escherichia coli and Pseudomonas fluorescens. Current Microbiology,36 (6), 341-347. doi: 10.1007/s002849900320 | ||
| + | </p> | ||
| + | <p><b>[2]</b>The fate of Gram-negative envelope during holin-endolysin lysis [Image]. (2000). Retrieved September 23, 2012, from: http://dx.doi.org/10.1016/S0966-842X(00)01705-4 | ||
| + | </p> | ||
| + | <p><b>[3]</b>Tran TA, Struck DK, Young R. (2007). The T4 RI antiholin has an N-terminal signal anchor release domain that targets it for degradation by DegP. Journal Of Bacteriology, 189 (21), 7618-7625. doi: 10.1128/JB.00854-07. | ||
| + | </p> | ||
| + | |||
| + | <p> <b>[4]</b>Christos G. Savva, Jill S. Dewey, John Deaton, Rebecca L. White, Douglas K. Struck, Andreas Holzenburg, Ry Young. (2008). The holin of bacteriophage lambda forms rings with large diameter. Molecular Microbiology, 69 (4), 784-793. doi: 10.1111/j.1365-2958.2008.06298.x | ||
| + | </p> | ||
| + | </FONT> | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
| + | |||
| + | </p> | ||
| + | </span> | ||
| + | </div> | ||
| + | |||
| + | |||
| + | </div> | ||
| + | </div> | ||
| + | </div> | ||
<!-- End Main --> | <!-- End Main --> | ||
| - | + | <!-- Footer --> | |
| - | <!-- Footer --> | + | |
<div id="footer"> | <div id="footer"> | ||
<div class="shell"> | <div class="shell"> | ||
<p class="left">Copyright © 2010 RightDirection. All rights reserved. Designed by <a href="http://chocotemplates.com" target="_blank" title="The Sweetest CSS Templates WorldWide">Chocotemplates.com</a></p> | <p class="left">Copyright © 2010 RightDirection. All rights reserved. Designed by <a href="http://chocotemplates.com" target="_blank" title="The Sweetest CSS Templates WorldWide">Chocotemplates.com</a></p> | ||
<p class="right"> | <p class="right"> | ||
| - | METU IGEM 2012 | + | <a href="https://igem.org/Team.cgi?year=2012&team_name=METU">METU IGEM 2012</a> |
</p> | </p> | ||
<div class="cl"> </div> | <div class="cl"> </div> | ||
| Line 592: | Line 592: | ||
</div> | </div> | ||
<!-- Footer --> | <!-- Footer --> | ||
| - | |||
| - | |||
| - | |||
| - | + | </body></html> | |
| - | + | ||
| - | < | + | |
Latest revision as of 22:42, 26 September 2012
Kill Switch |
Overview
Kill switch is a safety system which is necessary to control the release of genetically modified organisms (GMOs) into the environment. Since we aimed to construct a biofilm with GMOs which will convert CO to CO2, we needed a kill switch that can prevent our system to cause any harm to the environment. In order to achieve that aim we used the kill switch designed by Berkeley 2008 iGEM Team (Bba_K112808) and also we tried to develop it.
How The System Works ?
In order to construct our kill switch system we needed a reagent that E.Coli will grow and in the absence of that reagent all the bacteria should die. For that purpose we prefered to use IPTG (Isopropyl-beta-D-thiogalactopyranoside) because it cannot be metabolised by E.Coli and that is why its concentration remains constant in the cell.[1] When IPTG is present in the cell it will bind to LacI which can be produced by the promoter J23116. Basal LacI expression is already present in E.Coli but at low levels and it is hard to control the system with that much LacI. Hence, we prefered to produce it with the help of J23116. Our kill switch system includes the expression of endolysin, holin, antiholin and their production is controlled with the initial concentration of IPTG in the environment. Actually cell lysis can occur without IPTG but the lysis rate will be high. By using IPTG we aimed to keep our system under control.
Endolysin-Holin System; Endolysin and holin are the proteins needed for the cell lysis. Endolysin is a lysozyme and its funtion is to hydrolyze the peptidoglycan bacterial cell wall but it needs holin to form pores on inner membrane, then it can reach periplasm and cell lysis occur.
The fate of the Gram-negative envelope during holin-endolysin lysis:[2]

In this figure holins are shown as clear ovals and endolysin is shown as notched circles.
- a-)Pre-Hole Configuration: Accumulation of holin is present in the inner membrane, while endolysin accumulates in the cytosol. Holin action can be prevented with the inhibitors (shown with star and black ovals).
- b-) Hole Configuration: When the holes are formed by holin in the inner membrane, endolysin can attack the murein which is important for cell lysis to occur.
Antiholin; It is the protein that blocks the function of holin and it is important for the regulation of cell lysis.[3]
In our system antiholin is constitutively produced by the promoter J23116 and when antiholin level is in between 1000-3000 molecules, the cell will die.[4] Actually,it is the number of free holin molecules that determines if the cell will die or not.
Modelling
KILL-SWITCH MODEL
The aim of kill-switch model is to predict the behavior of kill-switch mechanism in different IPTG concentrations. We first tried to model mechanism by the help of ODEs. Then we used SimBiology toolbox of MATLAB to simulate system.Introduction:
As can be seen from the diagram our circuit involves constitutive production of LacI. LacI Promoter is negatively regulated by LacI. The control mechanism of our system is inhibition of LacI by IPTG molecule. Since LacI is inhibited by IPTG its effect on LacI promoter changes with respect to IPTG concentration.
Holin and Endolysin expression is controlled by cI promoter which is negatively regulated by cI.
Our first assumption in the model is that when the free Holin molecules, which means number of Holin molecules minus number of Antiholin molecules, reach 3000[ref] cell lysis occurs.
Because cI concentration depends on the activity of LacI promoter, we can conclude that in the presence of IPTG the concentration of cI will be high and expression of Holin and Endolysin will be lowered. Due to the constitutive production of Antiholin, we can say that free Holin molecules will be lowered and chance of cell lysis to occur will drop. However, to understand the overall behaviour of system with respect to IPTG concentration we need to model and simulate the system.

Mathematical Model:
In our model, we included all processes shown below.
Equations for mRNA transcription









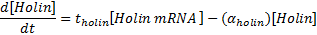


|
Parameter |
Description |
|
N |
Plasmid copy number |
|
Px |
Promoter Strength |
|
ax |
Degradation Rate |
|
Kx |
Disassociation (Equilibrium) Constant |
|
nx |
Hill Coefficient |
|
tx |
Translation Rate |
|
kx |
Binding on rate |
|
k-x |
Binding off rate |
|
Dx |
Diffusion rate |
Simulation Results
When the IPTG concentration is 0;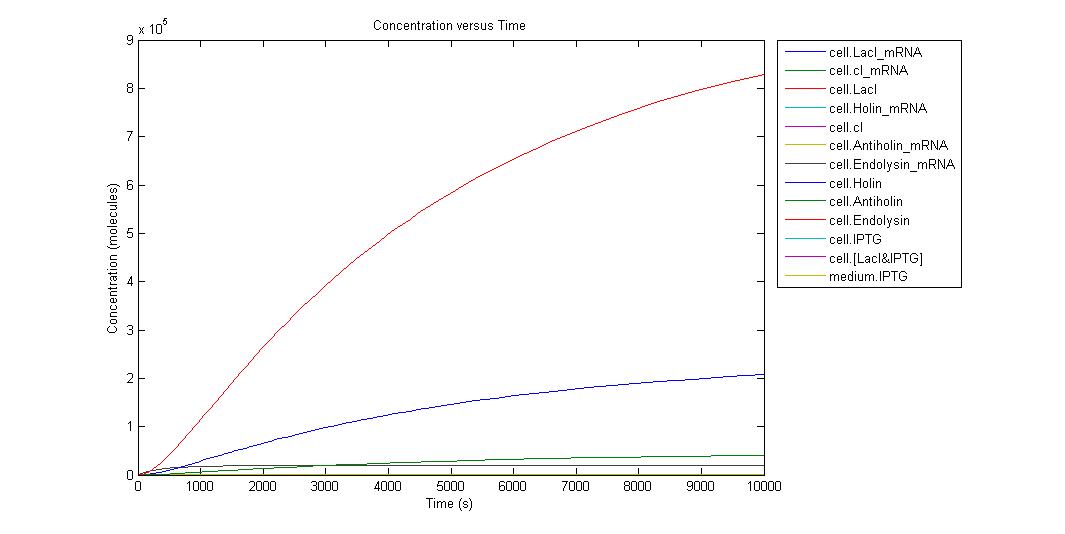
Individual graphs can be accessed here. Because the critical point in kill-switch is “Holin-Antiholin” molecules, we ran simulation in different IPTG concentrations and to understand result easier we select the outputs as Holin and Antiholin. Since translation rate of endolysin is more than Holin and Antiholin we can assume that there will be enough endolysin to make cell lysis occur. Also from the graph above we can understand that endolysin will be in sufficient amount.


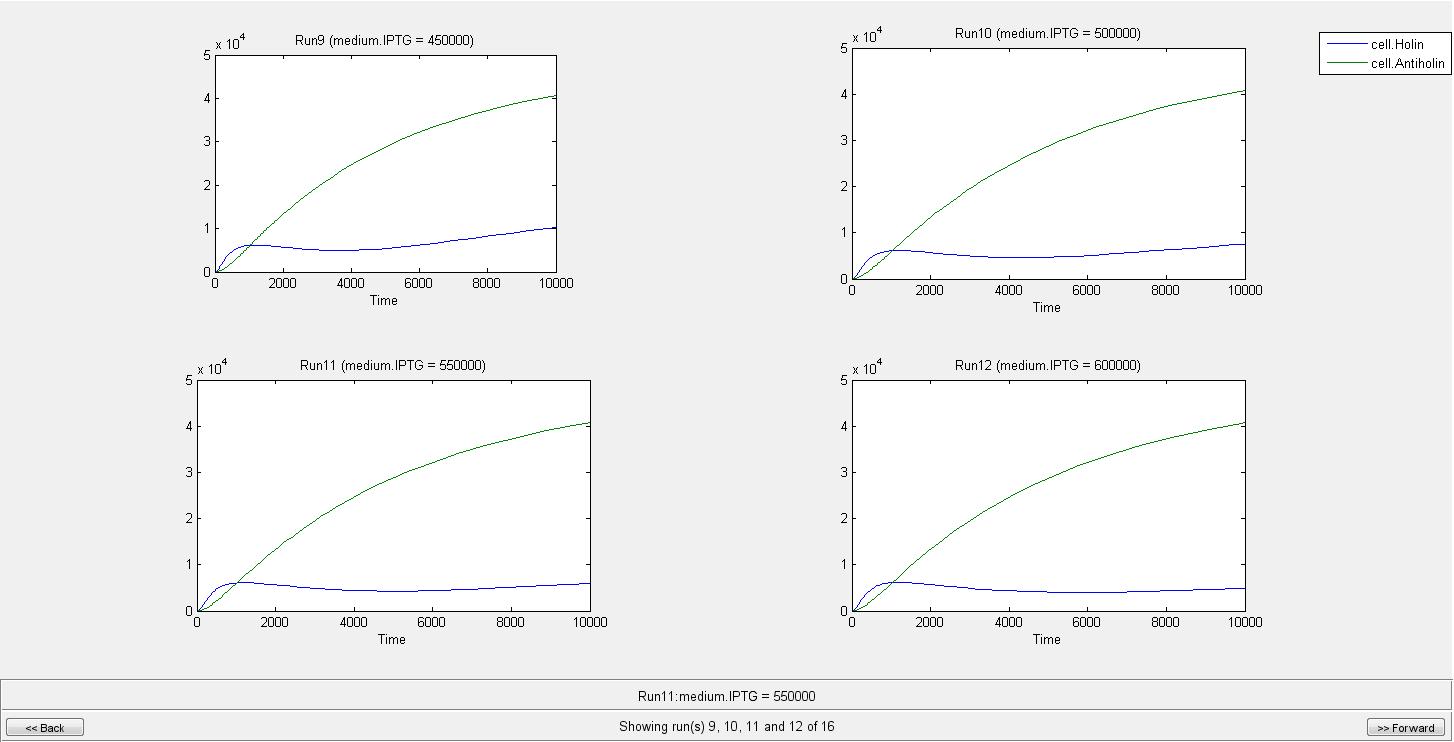

By the help of our model, we can conclude that when there is no IPTG molecule, cell lysis occur in 275 s. However when there is 100000 IPTG molecules, cell needs 2290 s to lyse. As can be seen from graphs until a very high IPTG concentration like in the Run14, the increase in Holin can eventually cause lysis. As a result of our model we concluded that after an IPTG concentration of 700000 molecule cell lysis will not occur. We are not considering the generation time of cell in this model since it can later be used for different organisms. If we consider e.coli with a generation time of 20 min, even in the medium with IPTG of 100000 molecules, it will divide before it can lyse.
When IPTG concentration is 700000;

Individual graphs can be accessed here.
Sensivity Analysis
Since the challenging part of modelling is choice of parameters, we conducted a sensitivity analysis to see how much result of our model depends on parameters. By the help of those, the critical values to be measured through experiments can be understood easily.






References
[1]Hansen LH, Knudsen S., Sorensen SJ. (1998). The effect of the lacY gene on the induction of IPTG inducible promoters, studied in Escherichia coli and Pseudomonas fluorescens. Current Microbiology,36 (6), 341-347. doi: 10.1007/s002849900320
[2]The fate of Gram-negative envelope during holin-endolysin lysis [Image]. (2000). Retrieved September 23, 2012, from: http://dx.doi.org/10.1016/S0966-842X(00)01705-4
[3]Tran TA, Struck DK, Young R. (2007). The T4 RI antiholin has an N-terminal signal anchor release domain that targets it for degradation by DegP. Journal Of Bacteriology, 189 (21), 7618-7625. doi: 10.1128/JB.00854-07.
[4]Christos G. Savva, Jill S. Dewey, John Deaton, Rebecca L. White, Douglas K. Struck, Andreas Holzenburg, Ry Young. (2008). The holin of bacteriophage lambda forms rings with large diameter. Molecular Microbiology, 69 (4), 784-793. doi: 10.1111/j.1365-2958.2008.06298.x
[1]Hansen LH, Knudsen S., Sorensen SJ. (1998). The effect of the lacY gene on the induction of IPTG inducible promoters, studied in Escherichia coli and Pseudomonas fluorescens. Current Microbiology,36 (6), 341-347. doi: 10.1007/s002849900320
[2]The fate of Gram-negative envelope during holin-endolysin lysis [Image]. (2000). Retrieved September 23, 2012, from: http://dx.doi.org/10.1016/S0966-842X(00)01705-4
[3]Tran TA, Struck DK, Young R. (2007). The T4 RI antiholin has an N-terminal signal anchor release domain that targets it for degradation by DegP. Journal Of Bacteriology, 189 (21), 7618-7625. doi: 10.1128/JB.00854-07.
[4]Christos G. Savva, Jill S. Dewey, John Deaton, Rebecca L. White, Douglas K. Struck, Andreas Holzenburg, Ry Young. (2008). The holin of bacteriophage lambda forms rings with large diameter. Molecular Microbiology, 69 (4), 784-793. doi: 10.1111/j.1365-2958.2008.06298.x
 "
"

