Team:Evry/GB
From 2012.igem.org
The GoldenBricks: A new and fast cloning technique for iGEM

Introduction
Context
Cloning is probably the most tedious and less rewarding task when engineering living entities in synthetic biology. With the development of the restriction enzymes and efficient DNA ligases, assembling small and large DNA pieces has become a common practice, but as everyone was using his own enzymes sets and assembly method, making large DNA constructs has always been challenging in itself. The first indisputable advance in the domain came with the invention of the first standardized DNA cloning method: the BioBricks []. The standard BioBrick format (BBF RFC 10) made possible to clone together all kind of genetic parts (or bricks) in a standard way, using only four different enzymes: EcoRI, XbaI, SpeI and PstI. But the real power of biobrick standardization is the fact that starting from this, it is possible to build database of DNA pieces that can be assembled in the very same way. The PartsRegistry today contains several thousands of described and characterized biological parts [] and is a sources of inspiration for thousands of biologists around the world.
If biobricks have opened new perspectives for engineering organisms and has proven to its efficiency over a decade, this technique is limited by the fact that all the fragments have to be assembled one by one and the task of making a construct is very difficult to automatize, because of the number of DNA purification step required. IGEMers today spend most of their time assembling genetic parts together, leaving little time for characterizing and testing their system.
Several cloning techniques capable of assembling multiple fragments at the time have been invented since the rise of the Biobrick format. The most popular one is probably the one known as the Gibson method [] that can be used to assemble up to four fragments at a time on a regular basis. The Golden Gate technique [] (>20 fragments at the time) and its new evolution, the MoClo [] (47 fragments in two times reported) are leading the way of another kind of cloning technique based on type II restriction enzymes. More efficient than the Gibson cloning, the Golden Gate also have the huge avantage that it can easily assemble parts of various sizes and the number of part assembled simultaneously goes far beyond what is reachable using PCR based technique. Moreover, it do not imply any DNA modifications, which reduces severely the probability of having a mutation at the ligation scar.
If these new techniques dramatically speed-up the assembly of DNA pieces, to our knowledge, no reported work has been carried in standardizing these methodologies. So far, biologists using Golden Gate have to engineer new overhangs and create a new library each time they are making a different kind of construct.
From Golden Gate and BioBricks to GoldenBricks
The BioBricks are a collection of parts that can be assembled one by one in a generic way. Golden Gate is a technique that enable the assembly of several tens of parts simultaneously. Taking inpiration from both technique, the 2012 Evry iGEM Team invented and developped a new methodology called GoldenBricks, merging the power of the two techniques while keeping the compatibility toward the old RFC 10 BioBrick format and preserving the possiblilty to make standard GoldenGate assembly simultaneously with GoldenBrick assembly.
GoldenBricks technique is a one-shot cassette construction using DNA parts coming either from a plasmid distribution or from PCR product, engaging up to seven (and possibly more) different parts. Moreover, GoldenBricks works for both eukaryotes and procaryotes DNA construction with the very same protocol. If a non classical assembly is required (as for testing the strength of a terminator) a new "split construction" method based on standard plasmids make possible to assemble parts in a non classical way.
Perspectives
Such technique offers great perspectives for the future of iGEM and of the parts registry in general. First, it would make possible the fast and simple assembly of complete cassettes using less expensive equipment, less toxic chemical and less sequencing runs than the BioBrick assembly. It would make fast and efficient cloning accessible for both researchers as well as for less experimented users, such as high school iGEMers or biohackers thanks to the simplicity of its protocol. Second, the creation of large expression cassettes using this method goes cheaper and faster than synthesizing the construct at the present day, which would guaranty the interest for a DNA database compared to de-novo synthesis for the years to come. And last, this method is a lot more easy to automatize unlike the RFC 10 biobrick format.
Theory
Requirements for the development of the new standard
In the continuation of what we said previously and in order to stay in the progression of the methodology we have tried in this work to stay as close as possible to biobrick format, to keep GoldenBrick parts compatible with the RFC 10 standard assembly, while opening new perspectives to increase the speed and the easiness of cloning. These constraints imposed several requirements for this new standard.
First, the new assembly method should:
- Keep the compatibility with the standard RFC 10
- Be compatible with a database approach
- Minimize the number of different prefixes and suffixes
- Use the standard registry plasmids and negative cloning control
To improve the cloning speed, we would also like to:
- Enable one step Golden Gate cassette cloning
- Allow to check the construct with a single enzyme digestion
- Improve the compatibility with Gibson
- Provide a solution for non standard assembly
As we will demonstrate, the GoldenBrick format we have established meet all these requirement. To understand on what basis we have created it, we are now going to explain you the principles that led the design of this format before we analyze the sequences of the GoldenBrick prefixes and suffixes and discuss of what can be done with this technique.
A brief introduction on type II restriction enzymes
Golden Gate cloning, (also known as type II restriction enzyme cloning) rely on a specific and very diverse familly of enzyme (the type II) very different from the one cloners are used to. The main difference from the cloning point of view is that they cut outside their recognition site no matter what sequence is present. A very small subtype of them can be used for cloning purpose, since they cut only on one side of the enzyme in a very specific way. The ones used in Golden Gate cloning and MoClo are BsaI and BbsI.

Fig 2: The recognition pattern of BsaI
The fact that the enzyme cuts outside have two significant advantages:
- You can generate as many different overhang you want with a single enzyme within the same tube
- You cannot cut again when the part is ligated to something else
And because of the last point, you can in fact cut and ligate in the mean time in the same batch, without the need to purify anything which gives a lot better yeild and a lot less mutation than with traditional methods.

Figure 3: Principle of the Golden Gate cloning method
Since the small cut BsaI fragment can re-ligate with its mother strand, in order to improve the yield, the assembly is a lot more efficient when using a cycling between digestion and ligation phases in a thermocycler alternating between 37°C and 16°C, which drives the equilibrium towards the formation of the product.
Analysis of a classical synthetic biology constructions
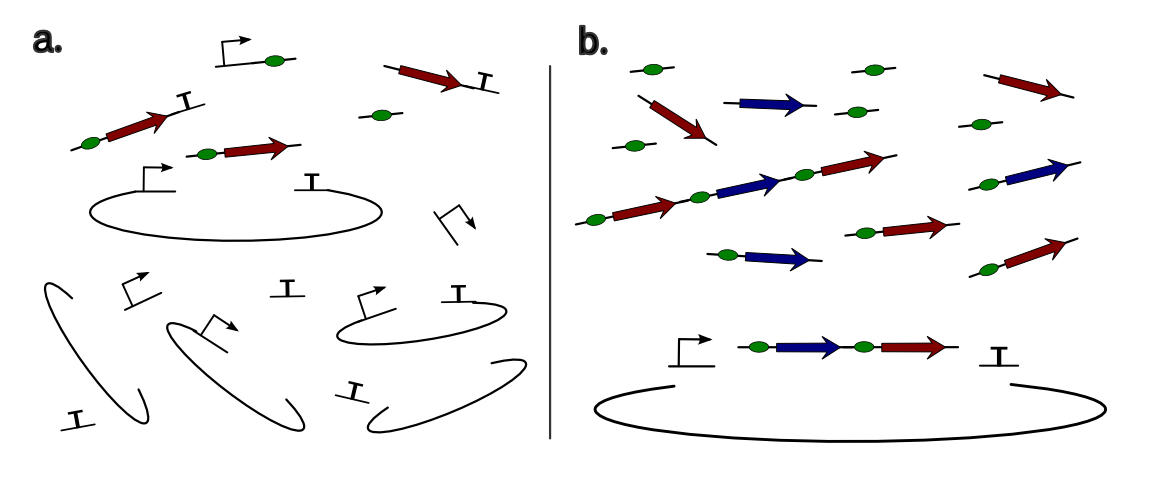
A traditional construct in synthetic biology is made of the repetition of the following elements:

Fig 4: Schematic of the different elements assembly in a standard synthetic biology device.
At the moment, GoldenBrick only allow the assembly of a single cassette at the time. In order to create the full system, the assembly of the different cassettes together have to be conducted afterwards with the biobrick assembly, Gibson assembly - that is very efficient to assemble long fragments - or as we will discuss, ligation independant cloning []. Focusing closer on a single cassette, we can notice that we have 4 different kinds of junctions:

Fig 5:In a single cassette, we can notice 4 different kinds of junctions. If we want the repetition of the {RBD-CDS} unit to be possible, the prefix of the RBS have to be compatible with the suffix of the gene.
As we can notice on this picture, there is 4 different kinds of junctions in a casette of device. In Golden Gate, the junctions have the form of a DNA overhang that we can freely engineer thanks to the capacity of Type II enzymes to cut outside its regognition site, no matter what sequence is there. Therefore, we should engineer 4 different overhangs. The first overhang is dedicated to the ligation of the plasmid suffix with the promoter prefix (OH1). Similarely, the 5th one is dedicated to ligate the terminator suffix with the backbone prefix (OH5). In a bacterial device, the scar between the RBS and the gene (OH3) has to be very well controlled because the distance between the two elements is critical for correct protein expression. This scar will remain the same than in the biobrick assembly, but it will be generated by a type II enzyme as for the others overhangs. Finally, the overhang that link the RBS prefix to the promoter suffix (OH2) and the protein suffix to the terminator prefix (OH4) have to be compatible to make possible the repetition of several {RBS-Protein} units in a single cassette (OH2=OH4). We will see later how to control this repetition.
To conclude, the necessity for 4 different overhangs implies to create 5 different prefixes and 5 different suffixes depending on the function of the DNA elements.
In addition, we would like the scar generated by the assembly of all the different elements to be digestible by a single enzyme. This would enable to control whether all the elements we intended to insert in the construct has been assembled before sequencing the casette. As we will see later this element will be critical for the selection of the clone that have inserted all the genes we want. This is why the overhangs 1, 2, 4 and 5 has been engineered in the middle of a BbsI site. When two pieces ligates, they recreate a BbsI restriction site, and we can check the correct insertion of a given gene in the construct by simply digesting with BbsI and checking for the size on a gel.
One last condition to fulfil to get a RFC 10 compatible brick after GoldenBrick cloning is to leave no illegal restriction site inside the brick after the assembly of the cassette. However, we should keep the as similar as possible the biobrick extensions around each elements leaving the 4 usuals enzyme restriction sites, so that the brick can be still assembled using biobrick method. The only drawback we didn't managed to overcome is that if several GoldenBrick parts are assembled with RFC 10 standard, they cannot be assembled together using GoldenGate afterwards, because the biobrick assembly will leave illegal BsaI site inside the casette.
The proposed new set of Golden Bricks extension
Having all these design principles in mind and meeting the requirements we have proposed, we designed the new GoldenBrick prefix and suffix with the following sequences:
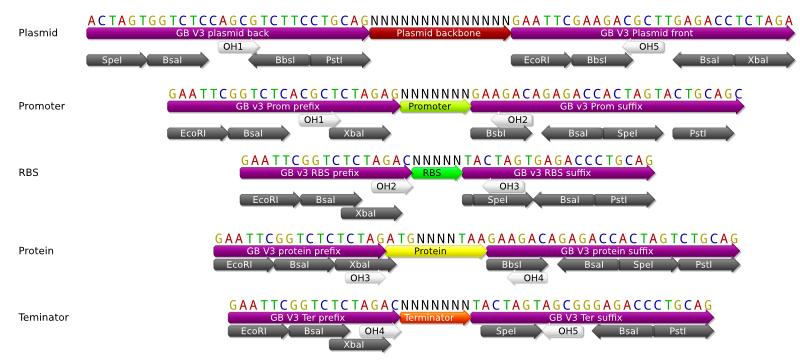
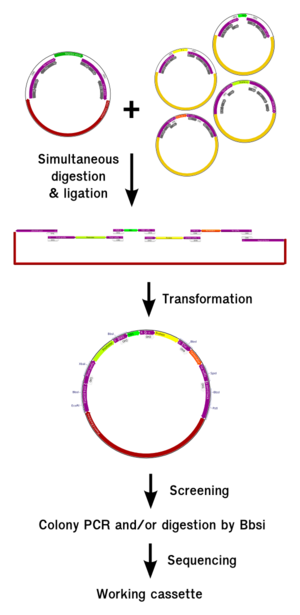
This design fulfil all the requirements enumerated before and ensure that the different elements will be assembled in the correct order. We also generate a scar that can be digested afterwards with the Bbsi enzyme, so that the correct insertion of the different elements can be check afterwards by doing a single digestion. This also enable the polymerization of the {RBS-CDS} units, as we will discuss in the next paragraph.
Control over the polymerization
The only difference with a standard GoldenBrick protocol is the capacity for the RBS-protein cassette to polymerize. This polymerization capacity makes the power of the technique because it enable to make polysistronic mRNAs no matter what gene is inserted. However, a control have to be achieved oved it in order to control when to create polysistrons and when we want a single gene to be inserted.
In order to acheive such a control, several parameters in the protocol will move the balance from mono-systrons to poly-sistrons. The first and the most obvious one is the stoechimoetry of the different elements. If the promoter and terminators are in large excess compared to the RBSs and the gene, the balance will be in favour of mono-sistrons. On the contrary, if the amount or RBSs and gene in dominant, the cassette will tends to contain two or more genes inserted inside.
On the other hand, the number of cycle and the ligation time will also influence the polymerization. Poly-sistrons are not likely to appear when the number of cycle is small and the ligation time short, but they will become more and more important as we increase these parameters.
Most of the work on GoldenBrick by our team is now focused in optimizing the protocol. We will release soon a set of standard protocols for mono and polysistrons optimized to reduce the polydispersity of the polymerization lenght.
Assembly procedure
Assembling parts using the GoldenBrick method is a lot easier than for BioBricks and also 5 to 10 times faster. We are now going to explain the procedure from the theoretical point of view. For the complete protocol, you should refers to the material and method section.
Because it requires a very small amount of DNA, the plasmid source can be taken directely from a DNA distribution suck as the partsregistry distribution, without requiring the need of a DNA preparation before. Once the plasmid are resuspended, the experimentalist mix them together in a mix containing the BsaI enzyme and T4 ligase and put them in the thermocycler for 5 hours. In the end, the product can be directely transformed and plated. A first round of screening sor the good polymerization length can be made using colony PCR with the standard VF2 and VR primers. If required, a second step of screening can be made after minipreping using BbsI. Given the very small rate of mutation, only two clones with the good digestion or colony PCR signature can be sent for sequencing; they will contain the correct construct with a very high probability.
This technique however requires to screen an important number of clones because we don't have a total control of what is assembling inside the cassette, because of the freedom we left for the RBS-Protein entities to polymerize. We will show in the next paragraph how we can gain a relative control over it. To reduce the costs of screening, we encourage the use of colony PCR oven mini-preping and digestion when possible.
Other advantage of GoldenBricks over any other standard cloning techniques
Non standard assembly and "split-construct" vectors
If you are willing ot create a non standard assembly or to control very precisely RBS-protein pairing over a polysistrons, you might like to create the construct in two times and assemble them together in a second step. This is why we have created the "split-construct" vectors. The split-contruct vectors are like the goldenbrick vectors except that they contain the OH2-OH5 or the OH1-OH4 overhangs instead of the OH1-OH5 overhangs as the GoldenBrick plasmid.
These vectors enable the assembly of shorter casette containing only a promoter, and a serie of RBS-protein for the "split-construct" 1 vector, or RBS-protein terminator for the "split-construct" 2 vector. This way, if a non standard biobrick assembly have to be carried in between the two pieces or if a long polysistron have to be created, they can be assembled using the standard EcoRI, XbaI, SpeI, PstI cloning, but several cloning step would have been saved by using GoldenBricks compared to a standard assembly.

GoldenBrick make standard part DNA shuffling possible
Aside from the dramatic shortening of the cloning time, the decrease of the costs and the easiness of GoldenBricks over Biobricks, one of the most noticeable improvement is undoubtedly the possibility to make DNA shuffling using a standard format. Lets take the example of a complex system such as a toogle-switch, in which you would like to optimize the expression of the protein repression of its two component to place the system in its working domain.
Using the Biobrick format, you will probably have to make tens of different constructs with different RBS to kind a condition in which the toogle-switch will work. Using GoldenBricks, you can make all of them in a single tube, in a single step. Moreover, you may eventually find a way to screen them directely after the cloning avoiding the need of purifying and sequence all the clones that couldn't work. The figure 11 shows in theory how one can proceed to such experiment design.

Many other use of DNA shuffling can be find in the literature especially for gene expression optimization and screening for working enzymes. We expect that DNA shuffling over standard parts is going to find many applications in the future of iGEM projects.
GoldenBrick is still compatible with Biobrick format
If the situation requires the part to be assembled with the standard RFC 10 format, the new GoldenBrick parts can be assembled the very same way than BioBricks. The only limitation is that BioBrick assembly of GoldenBrick parts leave a scar containing the BsaI restriction site inside, and the produced parts cannot be reassembled with other parts using Golden Gate. No simple way of fixing this issue has been found.
GoldenBricked plasmids are compatible with standard Golden Gate assembly
The GoldenBricked plasmids can be used to assemble contructions using classical Golden Gate. This option has been tested in our team and it works fine. (result not presented here)
GoldenBrick also assemble PCR products and shorten the assembly time
Someone than want to create a construct not having the time or the need of cloning his gene into a vector first can stil assemble them using GoldenBrick. Although this pracice wouldn't be encouraged for standards parts, it can become really usefull when trying libraries of different genes in order to find a working one. GoldenBricked parts in plasmid and PCR GoldenBricked parts can be mixed together and assembled the very same way. The user will just have to take care to submit his working parts afterwards. This is why we recommend to amplify new parts flanked by its GoldenBricks prefix and suffix, mix them with the GoldenGate assembly mix in one hand, and digest them in EcoRI and PstI on the other in order to ligate them in pSB1C3 forming the GoldenBrick.
Classical GoldenGate can be mixed GoldenBrick assembly to create fusion proteins
GoldenGate in general and GoldenBricks in particular are very efficient to assemble fusion proteins, because one can design every overhang he wants and therefore fuse proteins without making any scar. New fusion proteins can be created while beeing assembled in the standard GoldenBrick technique with custom made scar designed by the experimentalist. A new fusion protein made of several domains can be assembled in the middle of a polysistron without having to make the fusion protein first before cloning.
Finally, GoldenBricks works with eukaryotes chassis as well as with prokaryotes
As we have seen all along this article, this design of GoldenBricks have been design for procaryotes, but it can work very well for eukaryotes. In eukaryotes, promoters can include or not a 5'UTR in their sequence to enhance the protein expression. In the case there is no 5'UTR, we can use the same prefix and suffix than the prokaryote promoters and include the 5'UTR with the prokaryotes prefix and suffix.
In order to make polysistrons, we can use an Intermediate Ribosome Entry Site (IRES), cloned between an prokaryote RBS prefix and suffix.
Finally, the polyA and the polyA capping cassette with a polymerase terminator can be flanked by the prokaryotes terminators sites. In the case one don't want to include a polyA capping, he can use the "split construct" 1 or in the case there is not terminator, the one on the plasmid will take care of stopping the RNA polymerase extension.
Results
Construction of the library
In order to test the GoldenBrick technique efficiently, and for easiness of screening and making statistics, we have developed a set of GoldenBricked parts (see Material & Methods) with fluorescent proteins as a screening set. The parts have that we have created successfully are the following:
- K812050: A GoldenBricked version of pSB1C3 with J04450 as negative cloning control (status=sent)
- K812051: A GoldenBricked version of pSB1K3 with J04450 as negative cloning control (status=constructed)
- K812053: A GoldenBricked version of the strong RBS B0034 (status=sent)
- K812054: A GoldenBricked version of the RFP E1010 (status=sent)
- K812055: A GoldenBricked version of the terminator B0015 (status=sent)
- K812056: A GoldenBricked version of the pLac R0010 promoter (status=sent)
- K812057: A GoldenBricked of an sfGFP protein (status=constructed)
- K812058: A GoldenBricked of medium strenght RBS J61107 (status=constructed)
- K812059: A GoldenBricked of week RBS J61117 (status=constructed)
All the parts written here have been successfully constructed and sequenced and the ones marked as "sent" has been sent to the PartsRegistry.
Creation of a simple cassette
Now in the PCR machine!
Working with regular GoldenGate using GoldenBricks vectors
In our way of setting up new protocols and in order to assemble the fusion proteins we needed for our auxin system, we carried out two assemblies of fusion proteins using Golden Gate in our Golden Gated vectors. This paragraph reports the construction of the IaaH-Pep2A-IaaM fusion protein in the GoldenBrick plasmid K812050 using standard Golden Gate protocol. This protocol hadn't been optimized at the moment. However, out of 16 cloned on which we did colony PCR, 10 of them had the good size and out of the 3 sequenced, they were all correct.
This experiment demonstrates the possibility of doing Golden Gate in the GoldenBricks plasmids K812050 (and similarly K812051).
Material and methods
Construction of the library
The GoldenBricked parts were constructed amplifying the corresponding biobricks using primers flanked by the appropriate GoldenBricks extensions, using Phusion (Thermo Scientific) or Q5 (NEB) DNA polymerase in a 30 cycle reaction. The PCR product was PCR purified and then digested using FastDigest® EcoRI and PstI (Thermo Scientific). The standard plasmid pSB1C3 (or pSB1K3) were digested similarely. Both vectors and inserts were PCR purified and then ligated using T4 DNA ligase (Thermo Scientific) before being transformed with home made competent cells (chemical or electroporation).
The either red or non red colonies were minipreped and then digested with BsaI for screening. All the clones that had the correct digestion pattern were sequenced and gave the correct sequence.
The RBSs were synthetized in the form of two mathing oligos. A mixture of 10 µM of the two oligo was incubated at 98°C for 2 min and went down to 4°C with a ramp of -1°C/s. They were then PCR purified, digested and PCR purified again before beeing ligated in the same way.
Assembly protocol
The protocol given here han't been optimized yet. We are only starting the development of this new technology. It is given as a matter of indication. Some optimized protocol will be available soon.
About 75 ng of each plasmid (more accurate ratios will be optimized) were mixed together with 2.5 units of BsaI enzyme (NEB) and 15 units of T4 DNA ligase (Thermo Scientific) in a total volume of 15 µL of standard T4 ligation buffer. The tubes were then placed in the thermocycler with with the following steps:
- 37°C, 2 min (digestion)
- 16°C, 5 min (ligation)
- Goto 1, 50 times
- Option 1: if the construct does not contain any BsaI site: 50°C 5 min (reduce the background with a final digestion)
- Option 2: if the construct contains BsaI site: 16°C 2h (reconstitute the BsaI site)
- 80°C, 5 min (heat inactivation)
5 µL of the product was transformed with 50 µL of home made chemically competent cells and recovered for 1 hour. The cells were concentrated and plated.
Perspectives for GoldenBricks and the PartsRegistry
When the complete procedure will be established, our team will submit an BBF RFC request for the registry. If the RFC get accepted and if the tenant of the PartsRegistry thinks this is a valuable format, we can think of migrating the registry to this new format. This can be done in two ways: the first one is to ask every user to submit their new part in the new GoldeBrick format, and the second is to take advantage of the huge community working on the PartsRegistry and especially the iGEM comptetion. In order to migrate the parts faster, we can add in the requirements for the one of the medals for the teams to resubmit already exsiting parts in the new format. With an average of 200 teams in the years to come and asking each of them to resubmit 2 goldenbricked parts, we can cover the 2000 most useful parts within 2 or 3 years, because the teams will goldenbrick the parts they are interested in for their assembly, so they will probably always re-submit more than 2.
We beleive that implementing such a method in the partsregistry will speed up the assembly process and give a new shine to this database that severely suffers from the gene synthesis and PCR based methods competition. This will leave more time for researchers and iGEMers for characterizing the parts, and the partregistry will also benefits from an increase of parts characterization, which is very beneficial to the community. Overhall, the idea of standardized parts that tends to loose its strengh with the time will gain again in interest in the long run.
Conclusion
Our team this year have created a new cloning format for the PartsRegistry that dramatically speed up the cloning time and open new perspective such as simple protein fusion methods and DNA shuffling that were not possible using the current BioBrick format. At the moment, we are working very hard to demonstrate its possibilities and come up with an efficient and reliable protocol. If the process is found to be efficient, we will submit an RFC request to the PartsRegistry and propose that GoldenBrick progressively substitute to the current Biobrick format, which will renew the interest for people on standardized biological parts, seriously challenged by gene synthesis and PCR based methods today.
References:
- An introduction to agent-based modeling: Modeling natural, social and engineered complex systems with NetLogo, Wilensky, U., & Rand, W. (in press), Cambridge, MA: MIT Press
 "
"