Contents |
Miniprep of pSB1C3
Protocol: Miniprep
The slim tubes can be centrifuged in the machine in front of the "Gel hood", at 4000 rpm for 10 min. The fatter ones, in the E. coli centrifuge by the fridge (the tip can be left inside, since it floats).
Pellets resuspended with RNase containing buffer (Resuspension Buffer R3, from Invitrogen, equivalent to Buffer P1 from Qiagen, in Sowmya's box in the fridge). Note: keep the buffer in ice if you are not bringing it back to the fridge for some minutes.
We then use the QIAGEN QIAprep Spin Miniprep Kit with their [http://www.qiagen.com/literature/render.aspx?id=370 protocol] (page 22) and a microcentrifuge.
Minipreps of the circularized BioBrick plasmid containing the iGEM shipping vector (RFP) were made. However, there was far too much growth in the Falcon tubes, we probably should have put more chloramphenicol.
Nanodrop of the minipreps
Protocol: DNA Concentration Measurement
- Take a 6 µl aliquote of the DNA and put back the main DNA tube in the fridge.
- Go to the room by the E.Coli lab (LBTM, not on Friday morning!) with:
- The 6 µl aliquote
- A 10 µl pipet
- Optionally, the buffer you used for DNA elution (there might be some next to the machine).
- The machine is the NanoDrop Spectrophotometer.
- On the computer, click on "Nucleic Acid".
- Put a 2 µl drop of (nuclease-free) water on the machine's tip as you are asked to and measure.
- Clean tips (both sides) with a quarter of tissue.
- Add 2 µl of the buffer you use and click on "Blank".
- Clean tips (both sides).
- Add 2 µl of your DNA sample and click "Measure".
- Clean tips (both sides) with a tissue.
- Take 2 measurements per sample (for averaging).
- Print the report when you are done
- Click on exit.
The important numbers are:
- 260/280 ratio, must be > 1.8
- 260/230 ratio, must be > 2 (too big, > 2.5? , might mean too much salts)
- Of course the DNA concentration.
The Nanodrop showed dreadfully low concentrations. It was decided that we would redo a culture with a higher chloramphenicol concentration.
New overnight cultures
Overnight cultures of psb1-c3-rfp, psb1-c3 -lovTAP and pcep4-HA-readout were prepared.
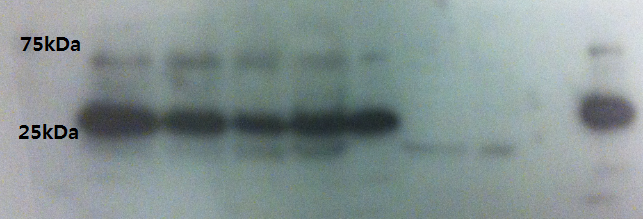
Western Blot result from 5.sep.12
- Double check VP16 antibody detection with 4.sep.12's result
 "
"