Team:TU Munich/Modeling/Gal1 Promoter
From 2012.igem.org



Contents |
Gal1 Promoter
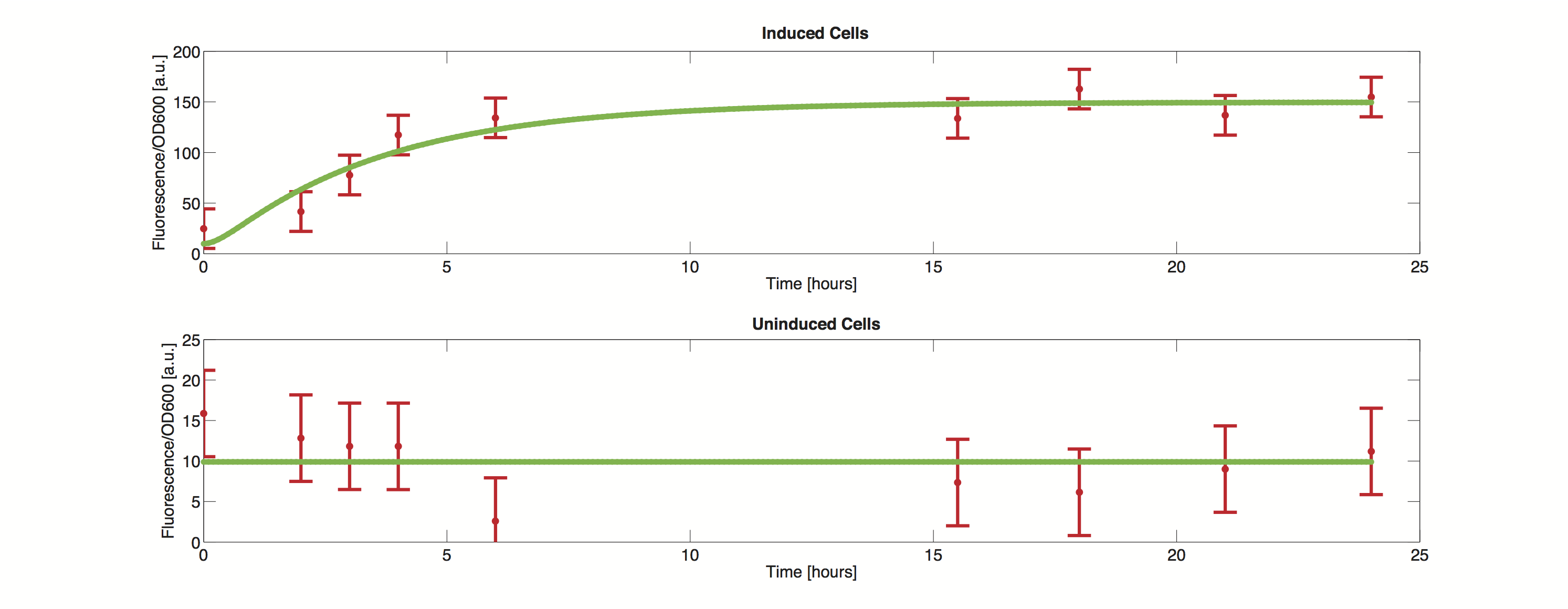
The Gal1 Promoter is the standard promoter for our pYES vector that is used in the expression of all ingredient pathways. As it is also part of the construct for the light switchable promoter, it is crucial to thoroughly characterize the kinetics of this promoter.
For details about the methods used see Team:TU_Munich/Modeling/Methods
Model Specification
Model Equations
We used a two species approach for mRNA and Protein concentrations. x1 and x2 represent the mRNA and protein levels for the induced promoter and x3 and x4 the respective levels for the uninduced promoter.
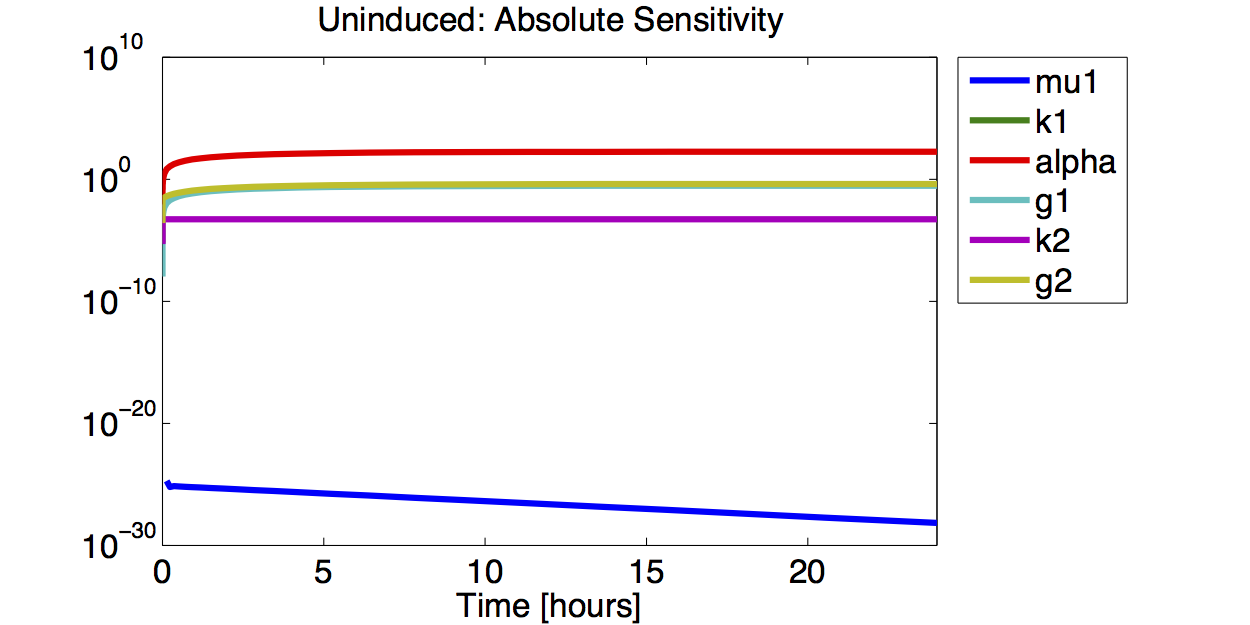
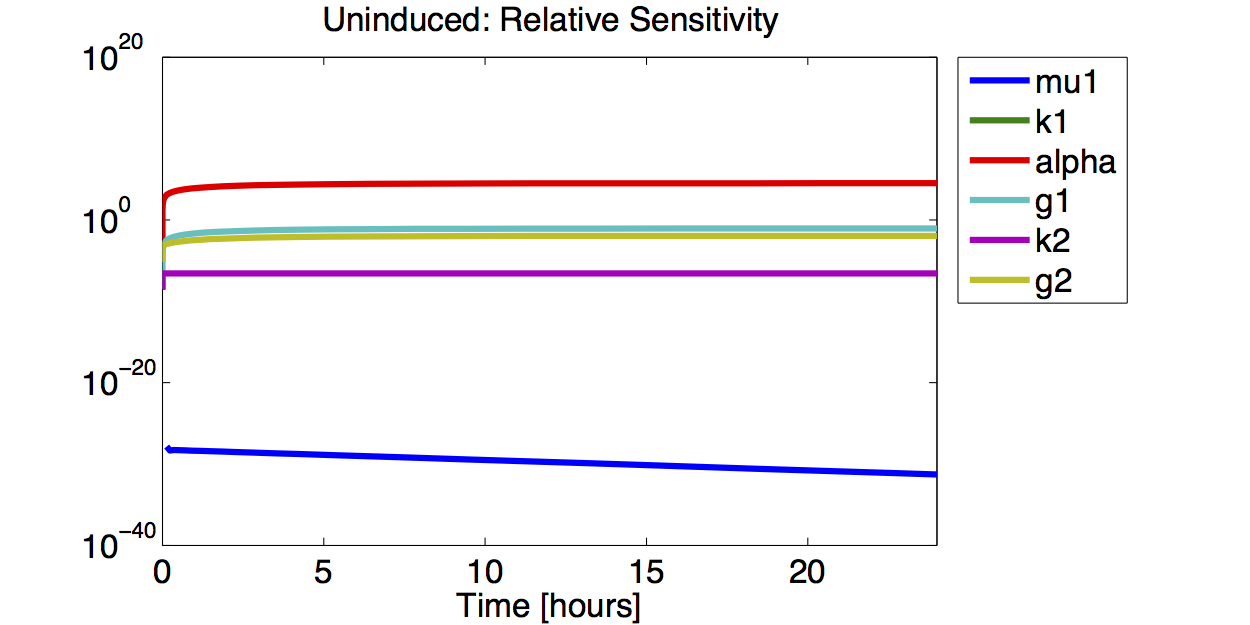
As no analysis was done with several galactose concentrations hence the hill function that normally models the response to the concentration was replaced by a single factor to improve identifiability.
Parameters
| Name | Description | Prior? | Best fit | Unit |
| μ | Scaling factor | NO | 114.9 | - |
| ki | Induced transcription rate | YES | 0.006758 | mol/h |
| α | Leaky transcription rate | NO | 0.0004784 | mol/h |
| γ1 | mRNA degradation rate | YES | 0.2892 | 1/h |
| k2 | Protein synthesis rate | NO | 165.9 | mol/h |
| γ2 | Protein degradation rate | NO | 3.1874 | 1/h |
| σ1 | Standard deviation for measured data of induced cells | NO | 19.55 | - |
| σ2 | Standard deviation for measured data of noninduced cells | NO | 5.337 | - |
The best fit is in a-posterior sense where the logarithm of the a-posteriori was optimised using the matlab script lsqnonlin. The script uses a Levenberg-Marquardt algorithm to find a minimum.
All fitted parameters are within realistic bounds.
The half life time of eGFP is a bit lower than expected. The literature states GFP should have a half-life time (inverse of the degradation rate) of over 20 hours. But there are several possible explanations for this:
- Certain GFP mutants are destabilizedm and thus have a significantly lower half-life time.
- The cells were not stored in the dark, hence photobleaching might lower the effectively measured half-life time as we only measure the non-bleached proteins.
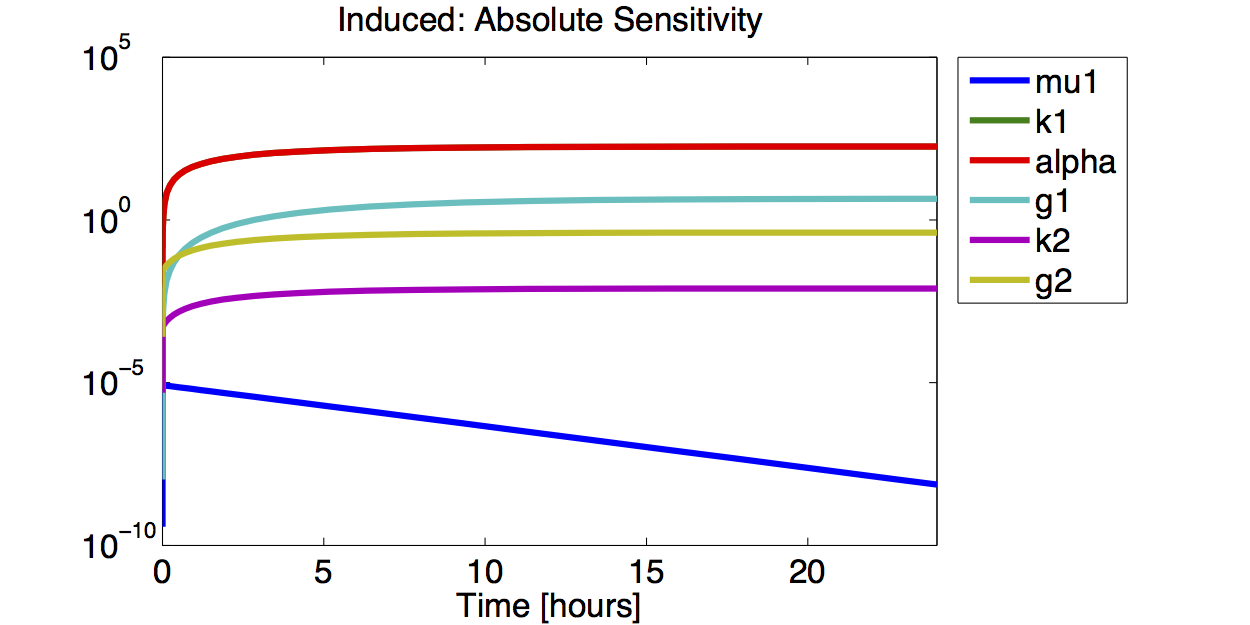
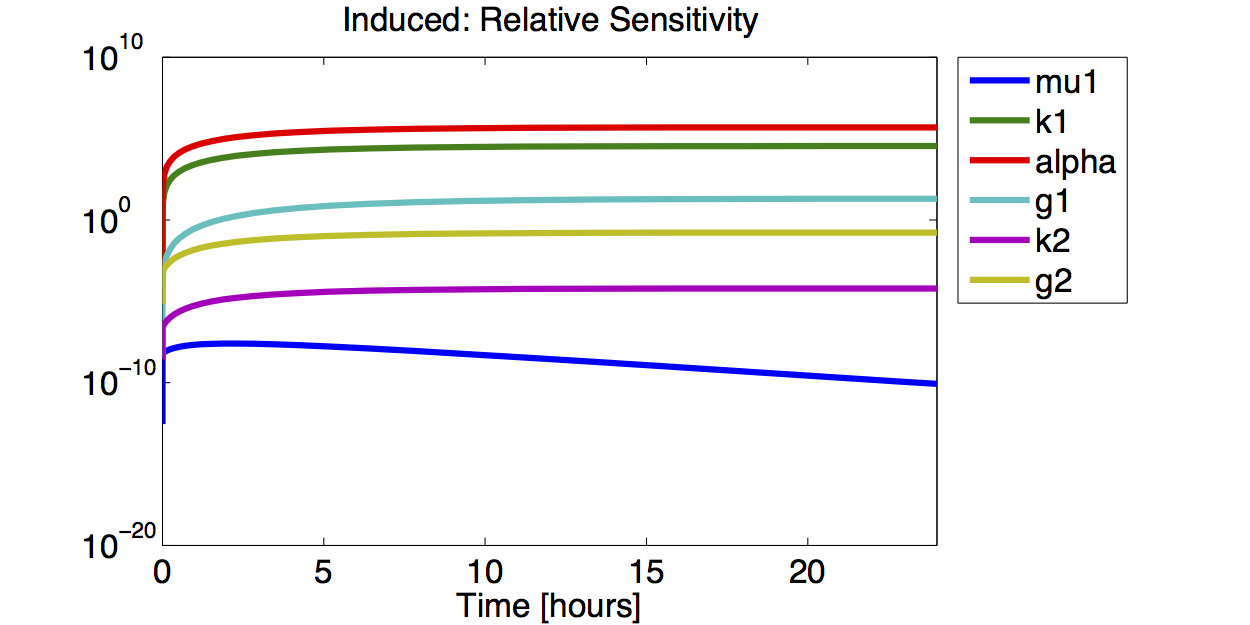
- The simulations show that the parameter set k2, %gamma;2 and μ is not precisely identifiable. This means that the best fit values are not necessarily significant.
Data
The methods utilized rely on a value for standard deviation, but only one experimental measurement was performed. Hence a value was infered during the optimization process. Thus the error bars in the plot do not reflect values from actual measurements but merely show how well the data can be approximated by the model.
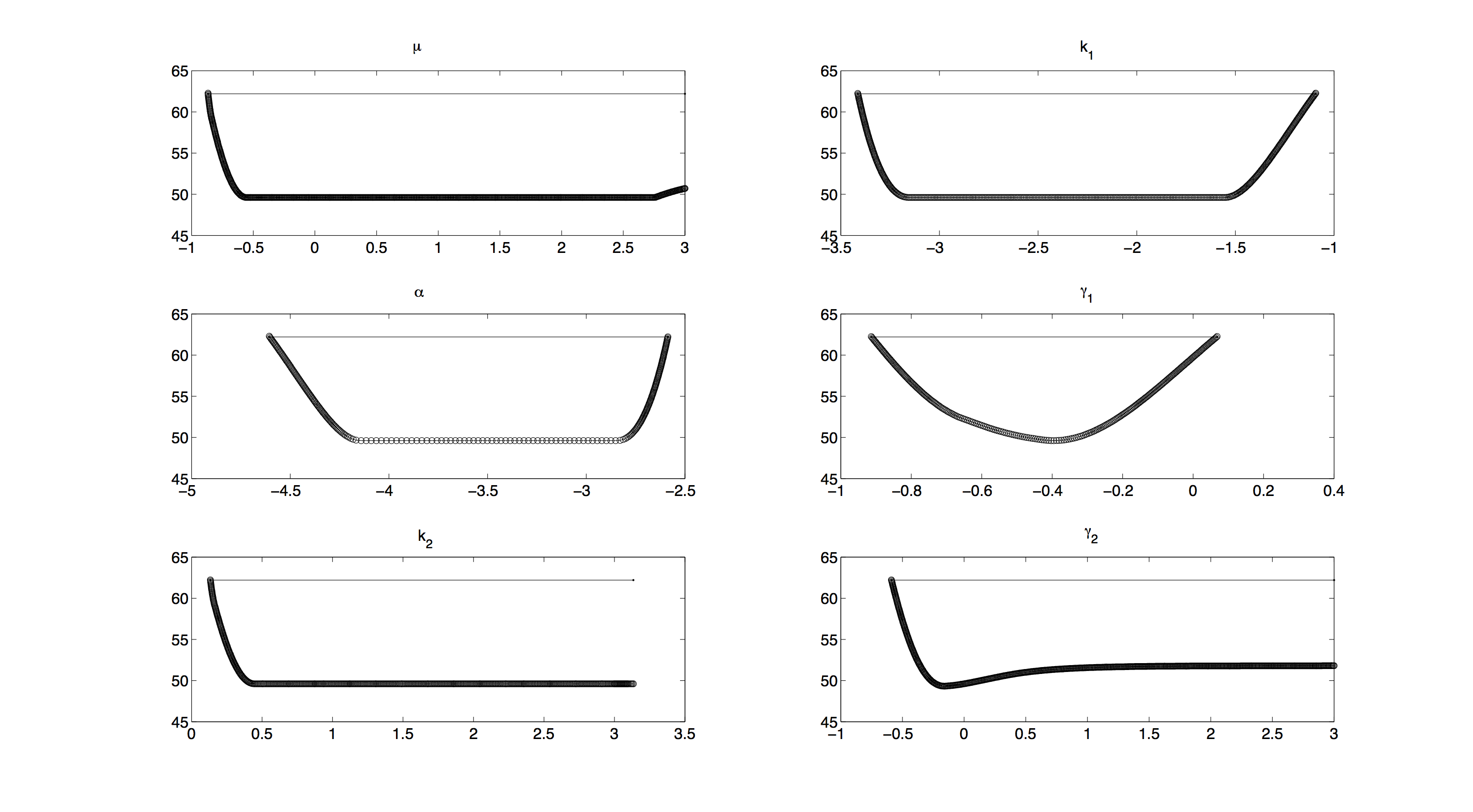
Profile Likelihood
For the profile likelihood analysis, the scripts provided by the Systems Biology group from TU Eindhoven[http://bmi.bmt.tue.nl/sysbio/software/pua.html] were used and modified to our needs.
All profiles in figure 2 have a convex shape, which means that we have no structural non-identifiabilities[http://bioinformatics.oxfordjournals.org/content/early/2012/02/21/bioinformatics.bts088] and all parameters are identifiable.
Including priors in further investigating will increase the accuracy of our measurements. Unfortunately, as computing profile likelihoods can become quite time consuming, we were not able to compute a profile likelihood with priors included.
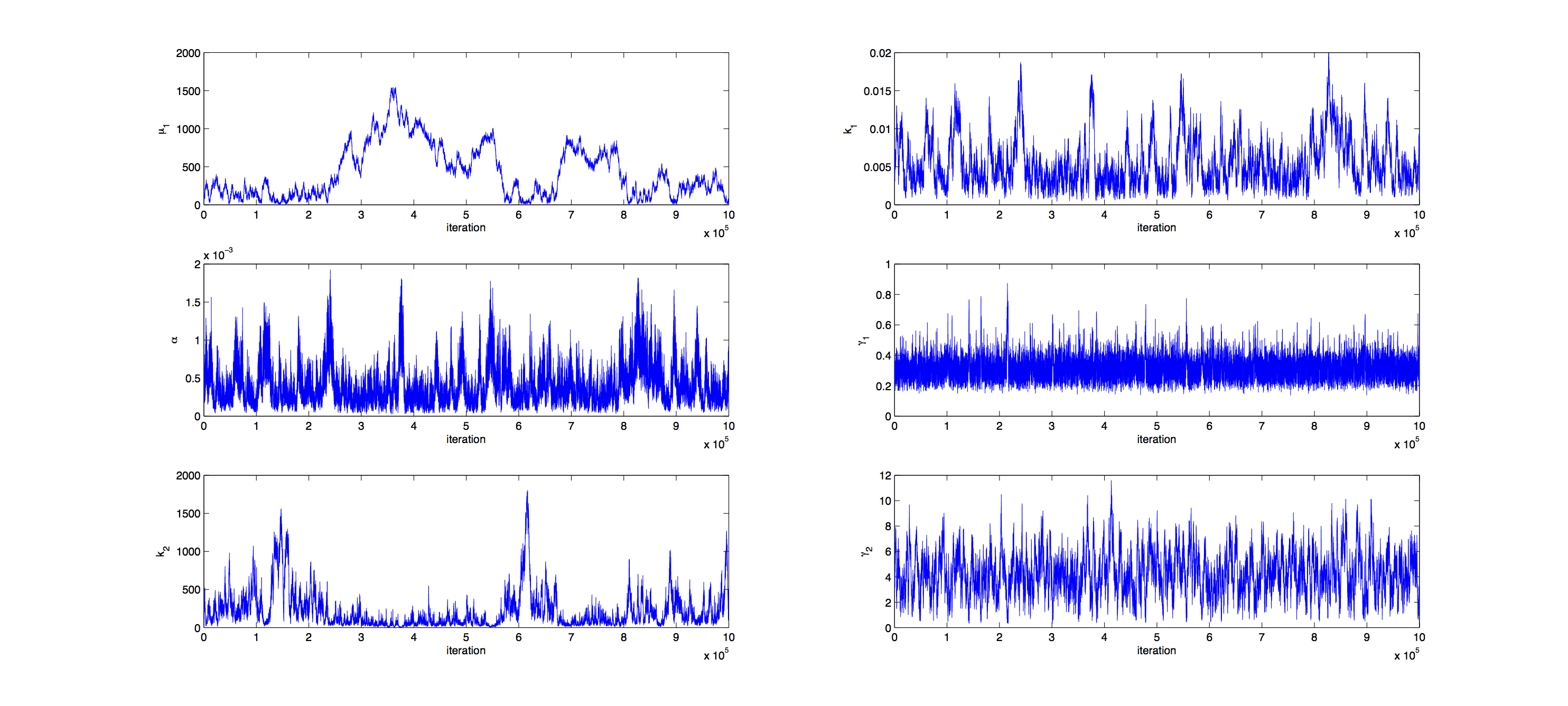
Markov Analysis
Analysis was performed with a Metropolis Hastings algorithm with a multivariate normal distribution as jumping distribution. 1000000 samples were generated and then thinned by a factor 1:100 to reduce correlation of the samples. The covariance matrix of previously generated samples were used to tune the jumping distribution, this resulted in an acceptance rate of 16%, so a little below the target of 23%.
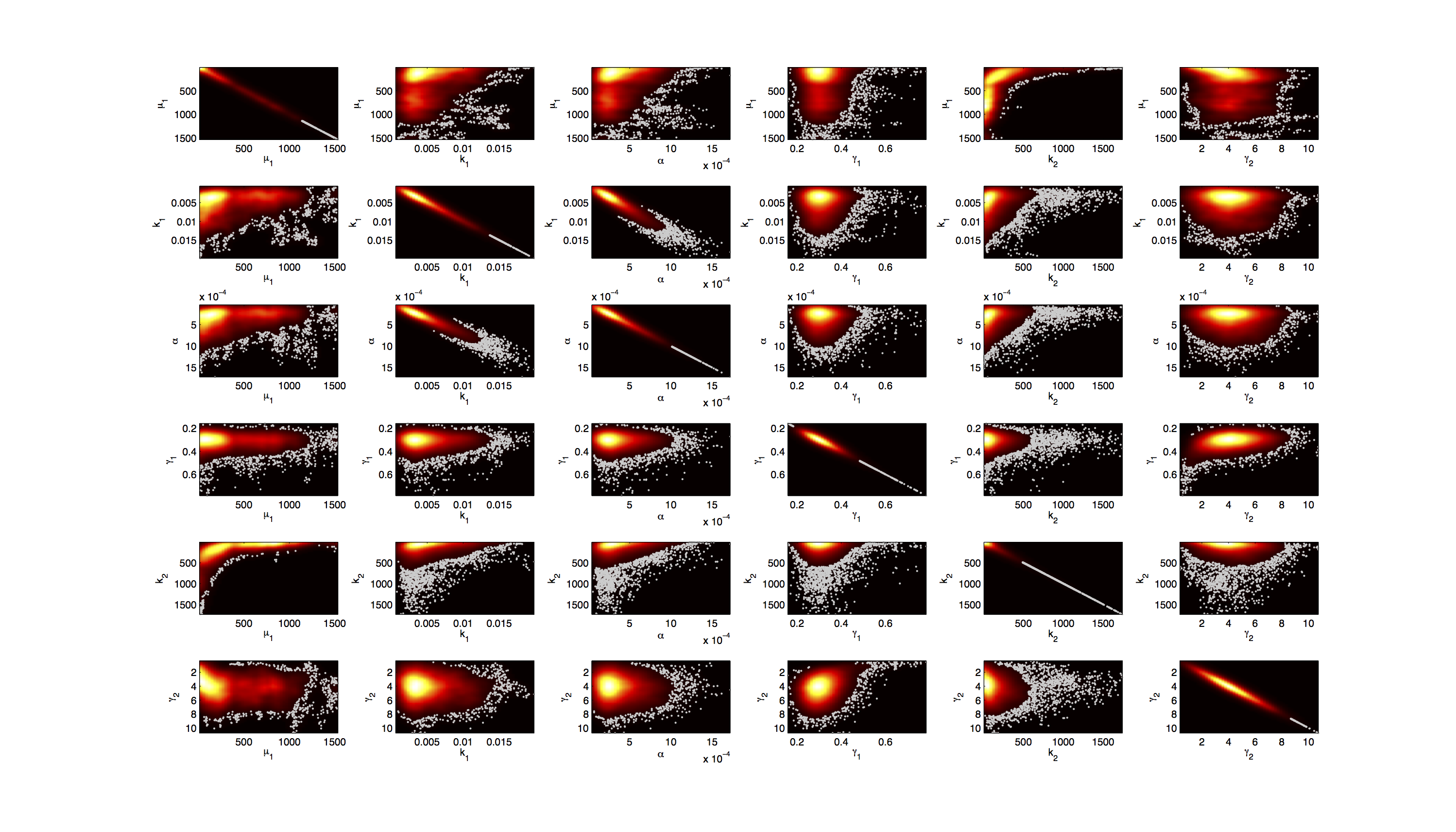
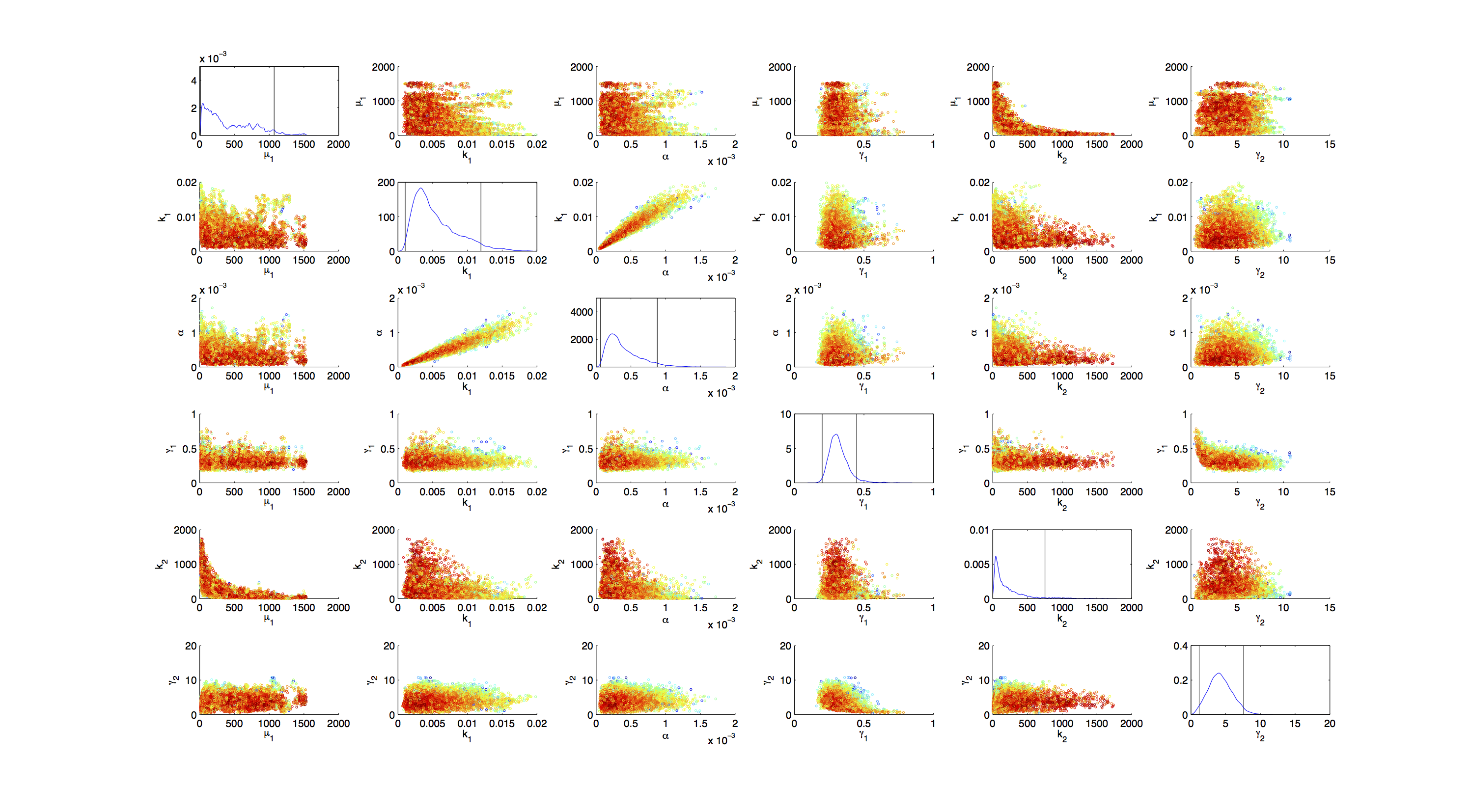
Joint Distribution
For the smoothed histogram plot smoothhist2d.m[http://www.mathworks.de/matlabcentral/fileexchange/13352-smoothhist2d] was used. The color indicates the density of samples. Black to dark red reflects a low density, yellow to white indicates a high density. Grey dots indicate samples identified as outliers by the script.
For the scatter plot further thinning of 1:1000 was necessary for scatter.m to be able to process the data. On the diagonal the kernel density estimation script kde.m[http://www.mathworks.com/matlabcentral/fileexchange/14034-kernel-density-estimator] was used to obtain a plot of the respective marginal densities. Here the color indicates the a-posterior probability of the respective sample. Low probabilities are mapped by blue to green color, High probabilities are mapped by yellow to red color.
Mind that due to the nature of the the utilized scripts, the orientation of the Y-Axis is flipped when comparing the two plots.
Credibility Intervals
As the Metropolis Hastings algorithm samples exactly from the target distribution, one can use the generated samples to find credibility intervals. The shown intervals are maximum density credibility intervals. They were obtained by computing the 1-α/2; and α/2 quantiles, with alpha being the missing percentage to 100%, that minimized the 1-norm of the distance between the two values.
| Param. | μ1 | k1 | α | γ1 | k2 | γ2 |
| 95% | [ 7.6525 ,203.2962] | [0.0011078 , 0.017724] | [8.0055e-05 , 0.001293] | [0.20397 , 0.45586] | [ 24.3219 , 954.8224] | [1.0073 , 7.3142] |
| 75% | [14.3725,116.5086] | [0.0020553, 0.0089666] | [0.00012838,0.00064765] | [0.24028 , 0.37610] | [126.3764 , 646.5652] | [1.8417 , 5.5854] |
| 50% | [14.7945 , 73.2214] | [0.0020910, 0.0058511] | [0.00017481,0.00044449] | [0.25771 , 0.33486] | [327.0597 , 639.2103] | [2.4019 , 4.6641] |
Covariance
| Param. | μ1 | k1 | α | γ1 | k2 | γ2 |
| μ1 | 9.5206278866 | 0.0005577146 | 0.0000392198 | 0.0249444112 | 12.9374858110 | 0.2451232424 |
| k1 | 0.0005577146 | 0.0000000334 | 0.0000000023 | 0.0000014843 | 0.0007684788 | 0.0000145516 |
| α | 0.0000392198 | 0.0000000023 | 0.0000000001 | 0.0000001037 | 0.0000536326 | 0.0000010162 |
| γ1 | 0.0249444112 | 0.0000014843 | 0.0000001037 | 0.0000662780 | 0.0341311274 | 0.0006455975 |
| k2 | 12.9374858110 | 0.0007684788 | 0.0000536326 | 0.0341311274 | 17.7991401047 | 0.3367804890 |
| γ2 | 0.2451232424 | 0.0000145516 | 0.0000010162 | 0.0006455975 | 0.3367804890 | 0.0063804120 |
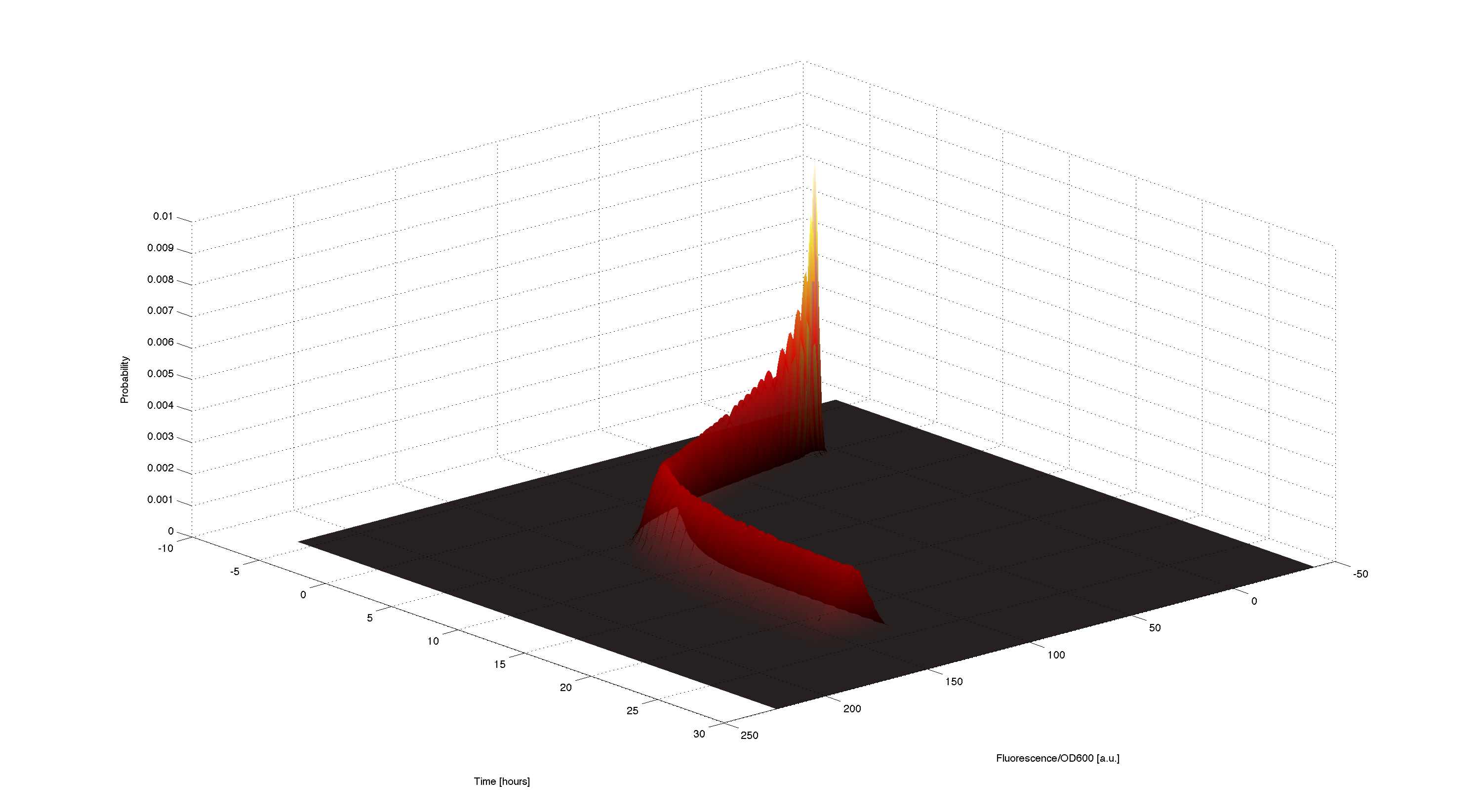
3D plot of resulting timecourse
Using the generated samples we can simulate the time course for all of them and use the 2d kernel density estimation script kde2d.m[http://www.mathworks.com/matlabcentral/fileexchange/17204-kernel-density-estimation/content/kde2d.m] to plot a wonderful distributed time-course of the simulated fluorescence.
Conclusion
Figures 3-5 all show a strong correlation between k1 and α, but as both marginal densities have a clear peak, this does not suggest an non-identifiability here.
 "
"