Team:TU Munich/Project/Genome Integration
From 2012.igem.org
(→Background and principles) |
(→Background and principles) |
||
| Line 19: | Line 19: | ||
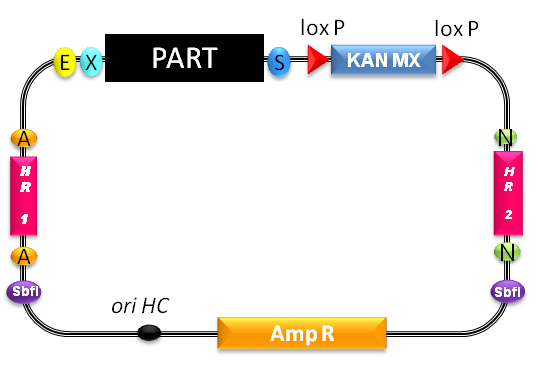
* "The part of interest, contained in the yeast integrative vector, will be inserted in the genomic region comprised between the two target sequences by homologous recombination, thus disrupting the original genomic DNA between them."[http://partsregistry.org/wiki/index.php/Part:BBa_K300986] | * "The part of interest, contained in the yeast integrative vector, will be inserted in the genomic region comprised between the two target sequences by homologous recombination, thus disrupting the original genomic DNA between them."[http://partsregistry.org/wiki/index.php/Part:BBa_K300986] | ||
| - | * The recombinant region includes the divergent GAL1/GAL10 promoter and | + | * The recombinant region includes the divergent GAL1/GAL10 promoter and several base pairs of the GAL1 and GAL10 ORFs. |
| - | + | * With SbfI linearized plasmids show a 10- to 50-fold higher integration efficiency than nonlinearized ones (Sherman F (1998), An Introduction to the Genetics and Molecular Biology of the Yeast Saccharomyces cerevisiae. University of Rochester Medical School, Rochester.). | |
| - | * | + | * As the plasmid itself contains no yeast ORI, colonies proliferating on G418 plates must have integrated the resistance gene. |
| - | * | + | |
== Idea == | == Idea == | ||
Revision as of 10:50, 23 September 2012



Contents |
Genome Integration
As we can't obey the letter of the German Purity Law (there is a zero tolerance policy concerning transgenic ingredients), we try our best to meet the spirit. Thus, it is unacceptable for us to work with antibiotics to keep up the selective environment. Since we can't work with auxotrophies in beer either, we have to make sure the yeasts don't get rid of the biobricks. The most promising way to accomplish a long lasting presence of our constructs is to achieve genome integration.
By means of genome integration we don't have to induce a selective stress in the brewing process and yet can be sure that the yeasts express our constructs properly - under constitutive promoters like TEF1 or TEF2 as well as ethanol inducible promoter or the light switchable promoter.
Background and principles
To accomplish genome integration we could resort to a preexisting biobrick [http://partsregistry.org/Part:BBa_K300001|Part:BBa_K300001].
Summary of principle:
- "The part of interest, contained in the yeast integrative vector, will be inserted in the genomic region comprised between the two target sequences by homologous recombination, thus disrupting the original genomic DNA between them."[http://partsregistry.org/wiki/index.php/Part:BBa_K300986]
- The recombinant region includes the divergent GAL1/GAL10 promoter and several base pairs of the GAL1 and GAL10 ORFs.
- With SbfI linearized plasmids show a 10- to 50-fold higher integration efficiency than nonlinearized ones (Sherman F (1998), An Introduction to the Genetics and Molecular Biology of the Yeast Saccharomyces cerevisiae. University of Rochester Medical School, Rochester.).
- As the plasmid itself contains no yeast ORI, colonies proliferating on G418 plates must have integrated the resistance gene.
Idea
- Plan to integrate mOrange, Thaumatin and Limonene and Brew beer with it
Cloning Scheme
Results
To show, that the stable integration worked, we picked 36 clones from the plates with yeasts transformed with the linearized integration vector plasmid (insert: BBa K300007). These 36 clones were put into non-selective YPD medium and passaged 4 times. After 48 hours, the last culture was used to inoculate an overnight culture. The OD of all candidates was measured. We had 5 negatives, probably due to mistakes in pipeting. The rest of the cultures were diluted to OD 0.4 and 100 ml of it were used to plate on selective (meaning G418+) petri dishes.
After the evolutionary pressure was lifted, the yeasts should've gone rid of the non-integrated vector. Another contact to G418 would not be tolerated by these yeast cells. Therefore carries every proliferating yeast cell the resistance decoded by the resistance gene on the integrational vector. The plates under lambda ____ show the typical fluorescence shown by mOrange.
Furthermore we will PCR the hell out of the yeasts to find out, where the integration took place (should be in the Gal-promoter region).
 "
"