From 2012.igem.org
I. Run Diagnostic PCR on Sample 3
- Mix according to the following table
| | A | B | C | D | E | F
|
| Reagent | (uL) | (uL) | (uL) | (uL) | (uL) | (uL)
|
| water | 33.5 | 34.5 | 36 | 36 | 33.5 | 34
|
| 5x Phusion HiFi buffer | 10 | 10 | 10 | 10 | 10 | 10
|
| 10 mM dNTP mix | 1 | 0 | 1 | 1 | 1 | 1
|
| 10 uM primer (F) | 2.5 (p3f_2) | 2.5 (p3f_2 | 0 | 2.5 (p3f_2) | 2.5 (p3f_2) | 2.5 (p3f_2)
|
| 10 uM primer (R) | 2.5 (p3r) | 2.5 (p3r) | 2.5 (p3r) | 0 | 2.5 (p3r) | 2.5 (p3r)
|
| Template | ECNR2 colony* | ECNR2 colony* | ECNR2 colony* | ECNR2 colony* | 0 | ECFI5 colony*
|
| Phusion HiFi polymerase | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0
|
*For these templates, I touched a colony with a sterile pipette tip and spread it on the bottom of the PCR tube before adding reagents
| Step | Temp (oC) | Time
|
| 1 | 94 | 4 m
|
| 2 | 94 | 30 s
|
| 3 | 53 | 30 s
|
| 4 | 72 | 1.5 m
|
| 5 | GOTO 2 | 7x
|
| 6 | 94 | 30 s
|
| 7 | 66 | 30 s
|
| 8 | 72 | 1.5 m
|
| 9 | GOTO 6 | 30x
|
| 10 | 72 | 5 m
|
| 11 | 4 | ∞*
|
- Store tubes on ice after PCR is finished
- Measure out 0.75 g of agarose and add to a 250 mL E-flask
- Add 75 mL of 0.5x TBE buffer and swirl to mix
- Cover flask with a Kimwipe and microwave for ~1 minute until clear
- Allow to cool to ~60 oC
- Immediately pour gel into tray with combs
- Allow to solidify, then remove the comb and tape and place the tray in the gel box with the wells closer to the black electrode
- Make sure it is submerged in TBE buffer
- Mix samples in PCR tubes with 10 uL buffer. Load 40 uL into one lane and the rest into another.
- Load ~6 uL on gel according to the following chart:
| Lane 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8
|
| NEB 2-log ladder | 2.2 (~10 uL) | 2.2 (~40 ul) | NEB 2-log ladder | 3.2 (~10 uL) | 3.2 (~40 uL) | 4.1 (~10 uL) | 4.1 (~40 uL)
|
-
- Close gel box and turn on power pack
- Run gel at 30 V for 25 min to stack gel
- Run gel at 75 V until the markers have reached ~3/4 down the gel
- Remove gel from box and cut along lanes to separate the 10 uL lanes from the 40 uL lanes.
- Return 40 uL lanes to the gel box
- Place 10 uL lanes in 200 mL of 0.5 ug/mL EtBr solution for 15 minutes
- Remove EtBr solution and wash with 200 mL of dH2O for 10 minutes
- View 10 uL lanes under UV to identify bands of interest
- Use a clean razor blade to nick gel where the bands are
- Match 40 uL lanes to the 10 uL lanes
- Using the 10 uL lanes as a guide, cut out the bands from the 40 uL lanes
- Weigh each gel slice in a colorless tube:
| LacZ (2.2) | CAT* (3.2) | tetr <4.1)
|
| 233 mg | 263 mg | 409 mg (treated as 400 mg)
|
- Add 3 volumes Buffer QG to 1 volume of gel
| LacZ (2.2) | CAT* (3.2) | tetr <4.1)
|
| 699 uL | 789 uL | 1200 uL
|
- Incubate in 50 oC water bath for 10-15 minutes until all gel has dissolved. Vortex to help mix.
- Note: The solutions were all yellow, OK to proceed
- Add 1 volume 100% isopropanol to samples and mix by inversion
| LacZ (2.2) | CAT* (3.2) | tetr <4.1)
|
| 233 uL | 263 uL | 409 uL
|
- Apply each sample to a QIAquick column 800 uL at a time. Centrifuge for 1 minute at 13000 rpm, discard flow-through, and repeat until all of the sample has passed through the column
- Add 500 uL of Buffer QG to the QIAquick columns and centrifuge for 1 minute, discard flow-through
- Add 750 uL of Buffer PE to the QIAquick columns and let the column stand for 5 minutes on the bench
- Centrifuge for 1 min and discard flow through
- Centrifuge again for 1 minute
- Transfer the columns to clean microcentrifuge tubes
- Elute DNA by adding 30 uL of Buffer EB to the center of the column and let stand for 5 minutes
- Bring samples and Buffer EB bottle upstairs to Take3 plate reader
- Add 2 uL of each sample according to the following chart:
| Blank (EB) | Blank
|
| Blank | Blank (diverging, ignored)
|
| LacZ | CAT*
|
| tetR | (empty)
|
- Use the program to blank the machine and measure sample concentrations
RESULTS
Concentration:
| Plasmid | Concentration (ng/uL) | Approx. volume (uL) | Approx. total (ug)
|
| LacZ | 11.468 | ~28 | 0.321
|
| CAT* | 35.256 | ~28 | 0.987
|
| tetR | 37.787 | ~28 | 1.058
|
V. AGE of Diagnostic PCR samples
- Measure out 0.5 g of agarose and add to a 250 mL E-flask
- Add 50 mL of 0.5x TBE buffer and swirl to mix
- Cover flask with a Kimwipe and microwave for ~1 minute until clear
- Allow to cool to ~60 oC and pour into flask for ethidium bromide (EtBr)
- Add 1 uL of EtBr stock to agarose and swirl to mix
- Immediately pour gel into tray with combs
- Allow to solidify, then remove the comb and tape and place the tray in the gel box with the wells closer to the black electrode
- Make sure it is submerged in fresh TBE buffer
- Mix samples as drops on a piece of parafilm
-
| Ladder (NEB 2-log) | Blank | Sample
|
*5 uL DNA ladder
*1 uL 6x Loading Dye | *8.3 uL dH2O
*1.7 uL 6x Loading Dye | *6.3 uL dH2O
*1.7 uL 6x Loading Dye
*2.0 uL DNA
|
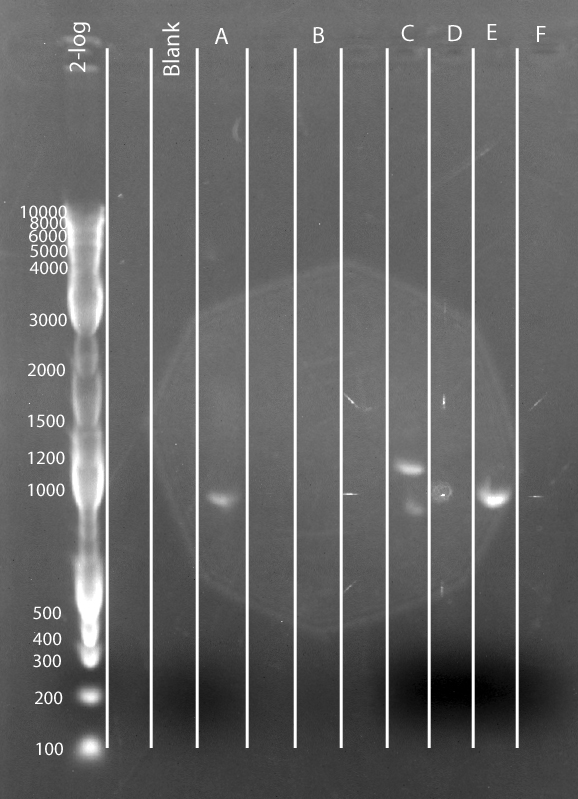
- Load ~8 uL (except ladder which is ~6 uL) on gel according to the following chart:
| Lane 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11
|
| NEB 2-log ladder | empty | Blank | A | empty | B | empty | C | D | E | F
|
- Store DNA at 4 oC
- Unusual loading pattern was due to poor loading
- Close gel box and turn on power pack
- Run gel at 30 V for 20 min to stack gel
- Run gel at 75 V until the markers have reached ~halfway down gel
-
Source: Media:Srk_2012-07-23_21hr_46min.tif
 "
"