Team:Tokyo Tech/Projects/PHAs/detail/index.htm
From 2012.igem.org
(→A .PHB production on colonies and preparation before confirmation with Nile red under UV) |
(→B.PHB production in cells and preparation before the confirmation with Nile blue A) |
||
| Line 194: | Line 194: | ||
| - | [[File:tokyotech PHA 7.png|250px|thumb|center| | + | [[File:tokyotech PHA 7.png|250px|thumb|center|Fig3-2-2. air permeable lids]] |
</div> | </div> | ||
Revision as of 03:20, 27 September 2012
Materials & Methods
Construction of pha-C1-A-B1 in Biobrick format
[Back to "Construction of phaC1-A-B1 in Biobrick format"]
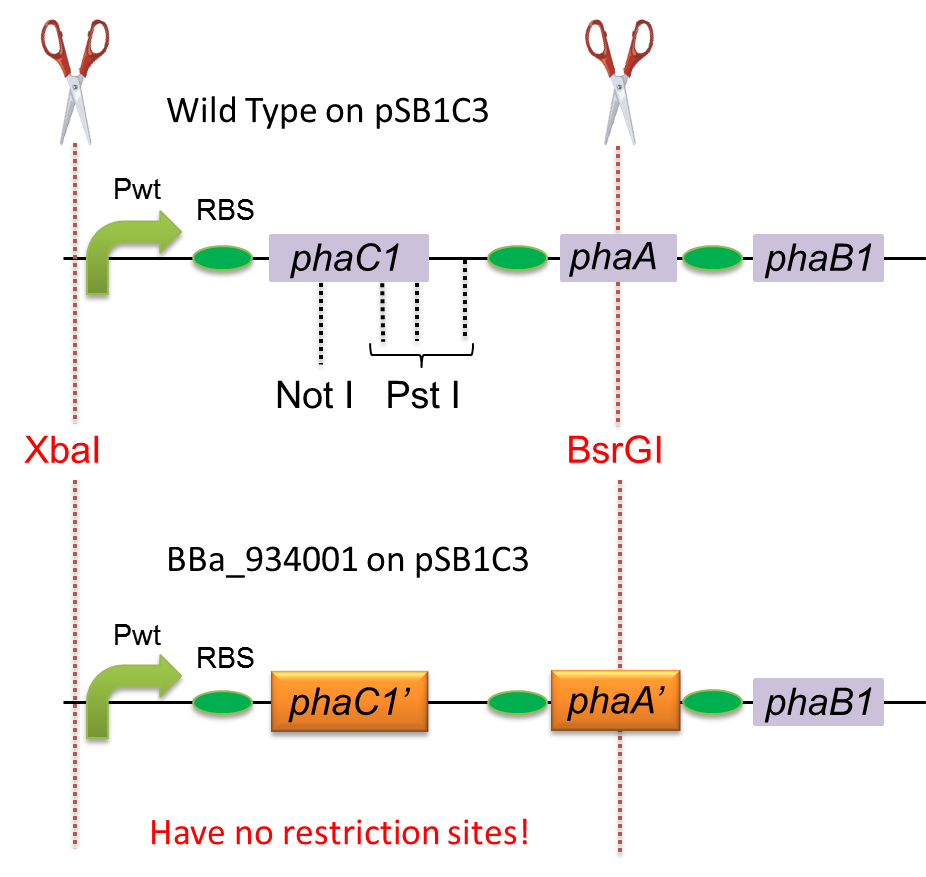
To construct a part that meets Biobrick format, we have modified the phaC1-A-B1 operon not to contain forbidden restriction enzyme sites. First, we cloned the wild type gene phaC1-A-B1 from R.eutropha H16 by using PCR and inserted the gene into pSB1C3. However, wild type phaC1-A-B1 gene sequence contains one NotI and three PstI recognition sites that are not allowed in Biobrick format. To get phaC1-A-B1 sequence without these recognition sites, we ordered the chemically synthesized DNA from IDT/MBL. In this chemically synthesized DNA, coding is optimized for E.coli. We used restriction enzyme XbaI (on pSB1C3) and BsrGI (on phaC1-A-B1) to insert sequence. That is to say, we got Poly[(R)-3-hydroxybutyrate] synthesizing gene in Biobrick format ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934001 BBa_K934001]). Fig3:construction of phaC1-A-B1
[Back to "Construction of phaC1-A-B1 in Biobrick format"]
Production trial of PHAs by past teams
[Back to "Construction of phaC1-A-B1 in Biobrick format"]
We introduce some attempts in the past iGEM to show how great our work is in iGEM.
| Part number | Description | States | experience |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K338003 BBa_K338003] | PHA Synthase Composite, Part 1/2 | Planning | |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K338004 BBa_K338004] | PHA Synthase Composite, Part 1/2 | Available | None |
They couldn’t prove that their engineered bacteria produced PHB according to the team wiki.
| Part number | Description | States | experience |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K342001 BBa_K342001] | Pha C (poly-β-hydroxybutyrate polymerase) | Available | None |
They registered and submitted a part, which contains only phaC sequence, as worked. But the team wiki shows that the part doesn’t meet the standard which is required in iGEM because they couldn’t cut their part with XbaI.
| Part number | Description | States | experience |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K282000 BBa_K282000] | phaAB | Available | None |
| Part number | Description | States | experience |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K089001 BBa_K089001] | phaA gene | Planning | |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K089002 BBa_K089002] | phaB gene | Planning | |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K089003 BBa_K089003] | phaC gene | Planning |
| Part number | Description | States | experience |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K125801 BBa_K125801] | RBS-phaA | Available | None |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K125802 BBa_K125802] | RBS-phaB | Planning | |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K125803 BBa_K125803] | RBS-phaC | Available | None |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K125804 BBa_K125804] | RBS-phaE | Available | None |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K125501 BBa_K125501] | phaA BioPlastic polyhydroxybutyrate synthesis pathway (origin PCC6803 slr1994) | Available | None |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K125502 BBa_K125502] | phaB BioPlastic polyhydroxybutyrate synthesis pathway (origin PCC6803 slr1994) | Available | None |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K125503 BBa_K125503] | phaC BioPlastic polyhydroxybutyrate synthesis pathway (origin PCC6803 slr1994) | Available | None |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K125504 BBa_K125504] | phaE BioPlastic polyhydroxybutyrate synthesis pathway (origin PCC6803 slr1994) | Available | None |
| Part number | Description | States | experience |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K156031 BBa_K156031] | RBS + phaA + double terminator | Available | None |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K156033 BBa_K156033] | RBS + phaB1 + double terminator | Available | None |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K156034 BBa_K156034] | RBS + phaC1 + double terminator | Available | None |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K156012 BBa_K156012] | phaA (acetyl-CoA acetyltransferase) | Available | None |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K156013 BBa_K156013] | phaB1 (acetyacetyl-CoA reductase) | Available | None |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K156014 BBa_K156014] | phaC1 (Poly(3-hydroxybutyrate) polymerase) | Available | None |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K156021 BBa_K156021] | Promoter + RBS + phaA + double terminator | Available | None |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K156018 BBa_K156018] | Promoter + RBS + phaA | Planning | |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K156019 BBa_K156019] | Promoter + RBS + phaB1 | Planning | |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K156020 BBa_K156020] | Promoter + RBS + phaC1 | Planning | |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K156022 BBa_K156022] | Promoter + RBS + phaB1 + double terminator | Planning | |
| [http://partsregistry.org/wiki/index.php?title=Part:BBa_K156023 BBa_K156023] | Promoter + RBS + phaC1 + double terminator | Planning |
[Back to "Construction of phaC1-A-B1 in Biobrick format"]
Protocol
A .PHB production on colonies and preparation before confirmation with Nile red under UV
[Back to "4-1 Confirmation of PHB synthesized on colonies"]
1 Preparation of LB agar medium plate containing Nile red and Glucose
1.1 Autoclave a LB agar(final 40g/L) solution at 120 ° C
1.2 After the autoclave, add Chloramphenicol(final 25ug/ml), Nile red and glucose(final 20g/L) to the LB agar solution when it cools down.
1.3 Make LB agar medium plates with the mixture.
2 Transformation of E.coli strain JM109 with pSB1C3 plasmid containing phaC1-A-B1 into strain JM109
2.1 Thaw the competent cells JM109 at 4° C
2.2 Add the target DNA 3ul into 1.5ml tube, then add in 50ul the thawed competent cells.
2.3 Put the tube into ice for 15mins
2.4 42° C,30secs, heatshock
2.5 Add 160ul of SOC into the tube
2.6 Incubate the transformant at 37° C for 30mins
2.7 Spread the resulting culture on LB agar medium with a large cone rod.
2.8 Incubate the plate at 37° C for 36hrs then cells the plate into 4° C room for 2-3 days.
[Back to "4-1 Confirmation of PHB synthesized on colonies"]
B.PHB production in cells and preparation before the confirmation with Nile blue A
[Back to "4-2 Confirmation of PHB accumulated in cells"]
1 Production of PHB
1.1 Acquire one colony of the transformed strains (JM109) with a platinum loop
1.2 Culture the colony in LB solution for 16hrs at 37 ° C
1.3 Measure LB medium (final 2.5%) and add it to each Erlenmeyer flask inside clean bench.
1.4 Add distilled water(final 95%) to each Erlenmeyer flask and cover the flasks with four-folded aluminum foil.
1.5 Set all flasks into autoclave
1.6 Add Chloramphenicol(final 25ug/ml) and glucose solution (50%) (final 20g/L) after the medium is completely cooled.
1.7 Add the solution of cultured cells into each flasks and shaking culture with air permeable lids at 37 ° C for 96 hours.
2 Preparation before the confirmation (with Nile blue A) under fluorescent microscope
2.1 Collection of PHBs in JM109
2.1.1 Weigh empty 50ml falcon tube without lid and make a record.
2.1.2 Add some culture solution into each tube.
2.1.3 Set the tubes into centrifuge and make sure that the label faces outside.
2.1.4 4 ° C, 5000G, 10mins in centrifuge.
2.1.5 Remove the supernatant with electric pipettor then add culture solution and set in centrifuge again.
2.1.6 After adding all the culture solution and setting in centrifuge, remove the supernatant and add water, set in centrifuge again.
2.1.7 Remove the supernatant and add a little amount of water
2.1.8 Cover the tubes with double layers of parafilms and fully freeze them.
2.2 Freeze drying (lyophilization)
2.2.1 Poke several holes on the tubes’ parafilm with toothpick.
2.2.2 Set the tubes on the freeze drying machine.
2.2.3 Freeze dry for 3 days.
2.3 Stain PHB accumulated dried cells with Nile blue A before observation
2.3.1 Acquire dried cells after freeze drying
2.3.2 Put a small amount of cells on the slide glass
2.3.3 Add water on the cells and heat the slide glass immobilize the cells
2.3.4 Stain the cells with 1% Nile blue A solution (water) for 8 minutes
2.3.5 Wash excess Nile blue A with 8% acetic acid solution
 "
"