Team:Colombia/Project/Basics
From 2012.igem.org
(→The Basics) |
(→The Basics) |
||
| Line 5: | Line 5: | ||
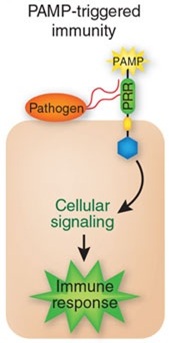
[[File:PAMP.jpg|left|150px|Upon pathogen attack, pathogen-associated molecular patterns (PAMPs) activate pattern-recognition receptors (PRRs) in the host, resulting in a downstream signaling cascade that leads to PAMP-triggered immunity (PTI).]] | [[File:PAMP.jpg|left|150px|Upon pathogen attack, pathogen-associated molecular patterns (PAMPs) activate pattern-recognition receptors (PRRs) in the host, resulting in a downstream signaling cascade that leads to PAMP-triggered immunity (PTI).]] | ||
| - | Our objective is to generate a genetically-modified bacteria "detect and alert" system, particularly as a defense aid for crop plantations against plant pathogens. Bacteria are built such that they can ''detect'' pathogen | + | Our objective is to generate a genetically-modified bacteria "detect and alert" system, particularly as a defense aid for crop plantations against plant pathogens. Bacteria are built such that they can ''detect'' pathogen associated molecular patterns ([http://en.wikipedia.org/wiki/PAMP PAMPs]), amplify this initial signal, and finally alert the plant by stimulating an early hypersensitive response against infection. We have also included a way for the bacteria to control their own population density using toxin-antitoxin modules. Using the bacteria ''Escherichia coli'' DH5α, the system will use two differently-modified plasmids. The first one will detect the designated PAMP and weigh such input for intensity and duration. If significant, it will also produce a signal molecule to engage the response of the second plasmid. Upon exposure to enough concentration of the molecule, the second plasmid will produce a systemic acquired response triggering hormone (salicylic acid) to activate the plant defenses, both attacking the pathogen as well as controlling the bacterial population growth. We strongly believe this biocontrol method may prove to become a powerful tool for farmers and crop workers everywhere. In addition, our constructed "detect and alert" biobricks could be useful to plentiful synthetic biology experiments and future iGEM teams. |
| + | |||
| + | We're currently implementing our design for the control of ''Hemileia vastatrix'' [https://2012.igem.org/Team:Colombia/Project/Problem#Coffee_rust_.28Hemileia_vastatrix.29 coffee rust] by recognizing fungal cell wall chitin, as well as the recognition of the ''Ralstonia solanacearum'' 3-hydroxypalmitic acid methyl ester (3-OH-PAME, a quorum sensing molecule) against [https://2012.igem.org/Team:Colombia/Project/Problem#Bacterial_Wilt_.28Ralstonia_solanacearum.29 bacterial wilt]. | ||
Bacteria are built such that they can detect chitin, an organic compound found in fungal cell walls, amplify this initial signal, and finally alert the plant by stimulating an early hypersensitive response against infection. We have also included a way for the bacteria to control their own population density using toxin-antitoxin modules. Using the bacteria ''Escherichia coli'' DH5α, the system will use two differently-modified plasmids. The first one will detect the presence of chitin and weigh such input for intensity and duration. If significant, it will also produce a signal molecule to engage the response of the second plasmid. Upon exposure to enough concentration of the molecule, the second plasmid will produce a systemic acquired response triggering hormone (salicylic acid) to activate the plant defenses, both attacking the fungi as well as controlling the bacterial population growth. We're currently investigating whether or not we can expand our detection plasmid for other plant pathogens such as ''Ralstonia''. We strongly believe this biocontrol method may prove to become a powerful tool for farmers and crop workers everywhere. In addition, our constructed "detect and alert" biobricks could be useful to plentiful synthetic biology experiments and future iGEM teams. | Bacteria are built such that they can detect chitin, an organic compound found in fungal cell walls, amplify this initial signal, and finally alert the plant by stimulating an early hypersensitive response against infection. We have also included a way for the bacteria to control their own population density using toxin-antitoxin modules. Using the bacteria ''Escherichia coli'' DH5α, the system will use two differently-modified plasmids. The first one will detect the presence of chitin and weigh such input for intensity and duration. If significant, it will also produce a signal molecule to engage the response of the second plasmid. Upon exposure to enough concentration of the molecule, the second plasmid will produce a systemic acquired response triggering hormone (salicylic acid) to activate the plant defenses, both attacking the fungi as well as controlling the bacterial population growth. We're currently investigating whether or not we can expand our detection plasmid for other plant pathogens such as ''Ralstonia''. We strongly believe this biocontrol method may prove to become a powerful tool for farmers and crop workers everywhere. In addition, our constructed "detect and alert" biobricks could be useful to plentiful synthetic biology experiments and future iGEM teams. | ||
Revision as of 18:36, 14 July 2012
Template:Https://2012.igem.org/User:Tabima
The Basics
Our objective is to generate a genetically-modified bacteria "detect and alert" system, particularly as a defense aid for crop plantations against plant pathogens. Bacteria are built such that they can detect pathogen associated molecular patterns ([http://en.wikipedia.org/wiki/PAMP PAMPs]), amplify this initial signal, and finally alert the plant by stimulating an early hypersensitive response against infection. We have also included a way for the bacteria to control their own population density using toxin-antitoxin modules. Using the bacteria Escherichia coli DH5α, the system will use two differently-modified plasmids. The first one will detect the designated PAMP and weigh such input for intensity and duration. If significant, it will also produce a signal molecule to engage the response of the second plasmid. Upon exposure to enough concentration of the molecule, the second plasmid will produce a systemic acquired response triggering hormone (salicylic acid) to activate the plant defenses, both attacking the pathogen as well as controlling the bacterial population growth. We strongly believe this biocontrol method may prove to become a powerful tool for farmers and crop workers everywhere. In addition, our constructed "detect and alert" biobricks could be useful to plentiful synthetic biology experiments and future iGEM teams.
We're currently implementing our design for the control of Hemileia vastatrix coffee rust by recognizing fungal cell wall chitin, as well as the recognition of the Ralstonia solanacearum 3-hydroxypalmitic acid methyl ester (3-OH-PAME, a quorum sensing molecule) against bacterial wilt.
Bacteria are built such that they can detect chitin, an organic compound found in fungal cell walls, amplify this initial signal, and finally alert the plant by stimulating an early hypersensitive response against infection. We have also included a way for the bacteria to control their own population density using toxin-antitoxin modules. Using the bacteria Escherichia coli DH5α, the system will use two differently-modified plasmids. The first one will detect the presence of chitin and weigh such input for intensity and duration. If significant, it will also produce a signal molecule to engage the response of the second plasmid. Upon exposure to enough concentration of the molecule, the second plasmid will produce a systemic acquired response triggering hormone (salicylic acid) to activate the plant defenses, both attacking the fungi as well as controlling the bacterial population growth. We're currently investigating whether or not we can expand our detection plasmid for other plant pathogens such as Ralstonia. We strongly believe this biocontrol method may prove to become a powerful tool for farmers and crop workers everywhere. In addition, our constructed "detect and alert" biobricks could be useful to plentiful synthetic biology experiments and future iGEM teams.
 "
"