Team:Calgary/Project/OSCAR/Bioreactor
From 2012.igem.org
Emily Hicks (Talk | contribs) |
|||
| Line 61: | Line 61: | ||
<p>Also, we mixed water, commercial NA’s, and hexadecane (model hydrocarbon) together in a small test tube to determine if we will indeed get a top layer of hydrocarbons like what we need. Indeed, this top layer of hydrocarbons was formed after two minutes of time to allow for separation.</p> | <p>Also, we mixed water, commercial NA’s, and hexadecane (model hydrocarbon) together in a small test tube to determine if we will indeed get a top layer of hydrocarbons like what we need. Indeed, this top layer of hydrocarbons was formed after two minutes of time to allow for separation.</p> | ||
| + | |||
| + | |||
| + | <h2>The Final System</h2> | ||
<h2>Next Steps</h2> | <h2>Next Steps</h2> | ||
Revision as of 17:25, 3 October 2012
[http://www.example.com link title]


Hello! iGEM Calgary's wiki functions best with Javascript enabled, especially for mobile devices. We recommend that you enable Javascript on your device for the best wiki-viewing experience. Thanks!
Bioreactor: The House of OSCAR

Introduction
A major goal of our project was to use genetically engineered bacteria to convert toxic organic compounds into recoverable hydrocarbons. In order to accomplish this goal our team needed to create a contained bioreactor system for our organisms to carry out these conversions. Because of the scale of the oil sands, such a vessel must be built on a large scale to accommodate the volume of tailings. Examples of such large scale bioreactors are found in wastewater treatment plants, tissue engineering, and even beer fermentation. All these types of systems need proper heat and oxygen exchange as well as suitable pH and agitation control, and for our system the addition of ideal flow rates, growth medium, and optimizing conditions for bacterial growth and biochemical conversion left us with many design considerations to take into account.
In addition to developing a design for the bioreactor that took these factors into account, we were also tasked with finding a way of extracting the produced hydrocarbon product from the bioreactor. Theoretically, the hydrophobicity and light density of the hydrocarbons should cause them to settle in the top layer of the solution. There are many methods that can be used to separate a certain layer into various components such as using a centrifuge or even various filters.
The bacteria we are working with in our system are safe, lab strains of Escherichia coli which have been engineered to contain genes that are found in naturally occurring tailings pond organisms. Even though they are harmless, in order to prevent escape into the environment and horizontal gene transfer we have implemented biological controls in the form of a genetic kill switch mechanism to prevent our organisms from surviving outside the bioreactor system. Secondly, because of their lab-strain nature, our organisms should in theory be out-competed in any natural environment due to the the high metabolic costs associated with the metabolic pathways we have engineered them to express. In light of the issue of containment with genetically modified organisms, we have designed our system with multiple levels of controls that range from biological to physical containment measures to keep our organisms contained.
Research
Before diving into a making bioreactor, we first had to research current solutions in the field. To help us with this phase, we read research papers on bioreactors, toured a wastewater treatment plant, interviewed graduate students in the field, and had weekly meetings with the supervisors and biologists on our team. The bioreactors at the wastewater treatment plant were contained in open systems since they used natural microbes. The reactor also contained an air sparger to oxygenate the solution and spun at an extraordinarily slow rate. This was one of the many bioreactor processes we looked into. Below are pictures from our trip!
Our Bioreactor Evolution
Throughout the summer we worked on creating a prototype of the bioreactor. Our ideal process would be a fed-batch system, where the reactors would be continually fed with more bacterial nutrients and fresh tailings. The fresh tailing would be added when the naphthenic acid concentration within the bioreactor is lower, thus allowing the bacteria to continually convert naphthenic acid to hydrocarbons. Additionally, the process would have to occur within an enclosed system to ensure its sterility.
The Prototype
We determined a few basic concepts that needed to be included in the prototype. For one, we needed to make our bioreactor a closed system in order to keep our solution sterile. This is necessary to allow only our bacteria (E. coli with an ampicillin resistance marker) to grow. Employing a closed system bioreactor is also necessary as a physical containment measure to confine our organisms to our system.
Next, we needed to use a batch system for our system to work at lab scale (for competition data). This means that we are allowing the entire process to occur, and then removing all of our solution when our reactions have come to completion. This batch will include an appropriate growth medium to optimize our organisms growth, as well as a compound. This opposes a continuous stir method, where product is removed at the same rate that biomass and nutrients are added. A continuous method would be more appropriate at an industrial scale (1000+ L tanks), where the bacteria will have enough time to convert NA’s in such large tanks.
Furthermore, we had to find a way to remove the hydrocarbons once they were produced. We decided to use a belt skimmer, similar to those used to help clean up oil spills. This method is useful because it allows us to run the belt to pick up hydrocarbons without having to remove the entire solution of the batch. This way the bacterial culture already present in the tank can be maintained in active culture to continuously produce more hydrocarbons. To ensure sterility the belt skimmer will have a UV light attached across the belt to ensure sterilization of any bacteria picked up by the skimmer before the hydrocarbons are skimmed from the bioreactor chamber.
Finally to ensure optimal bacterial growth and production of our desired product, we included both a sparger and a turbine in the bioreactor design. The purpose of the turbine was to mix the solution preventing bacterial cells and other heavier materials from settling at the bottom of the vessel. Continuous mixing of the solution would also ensure even nutrient and reactant distribution in the tank. The sparger oxygenated the solution, aeration which is necessary for our aerobic bacteria to thrive. When assembled together the turbine would be located above the sparger thus breaking each bubble from the sparger into smaller ones. In a thicker solution such as tailings, both the air sparger and turbine will agitate the solution thus mixing it and prevent heavier materials from settling at the bottom of the chamber.
Once we had these designs in place, we were able to start building models and presentation material. One of our goals was to have a computer animation of our design in motion. We were able to meet this goal by using programs called Maya and RealFlow. Maya is a complex and extremely versatile computer animation program used for many animated movies, including James Cameron’s “Avatar”. RealFlow is a particle-generating program, used primarily for creating fluid flow and fluid effects. Combining both of these programs, we created a seventeen second long video showing the basic idea of how our bioreactor will work. In addition to this, we made a functioning model of a tank with our turbine, belt skimmer, and sparger included inside. Our model is also a closed system, which will be brought to the competition to demonstrate to the judges.
Particle Simulation Using RealFlow2012
Open System Showing Separation of Hydrocarbon Layer
Closed System Showing Emulsified Hydrocarbons
Testing and Results
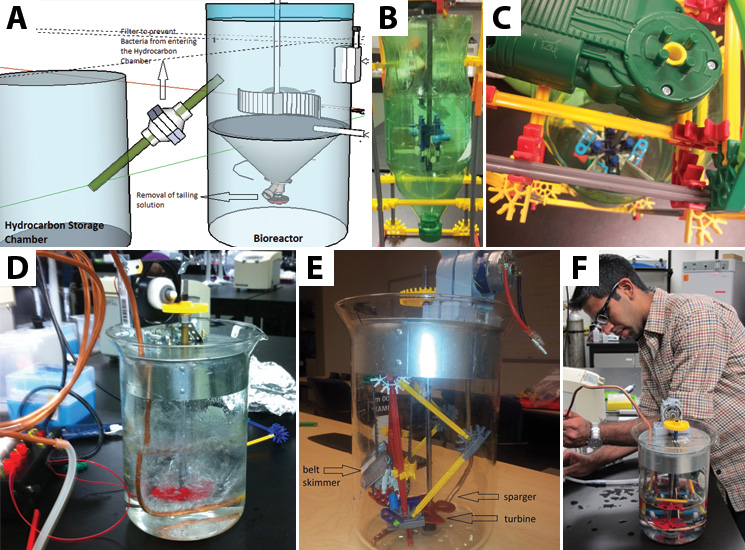
Using the physical models that we made, we were able to conduct experiments to help determine what will make our design most efficient. We received five different belt samples from a belt skimming company (Abanaki), so we conducted three different tests to determine which belt would work best for us. Our tests sought to find the belt that picked up the most oil, least tailing pond material, and least amount of bacteria.
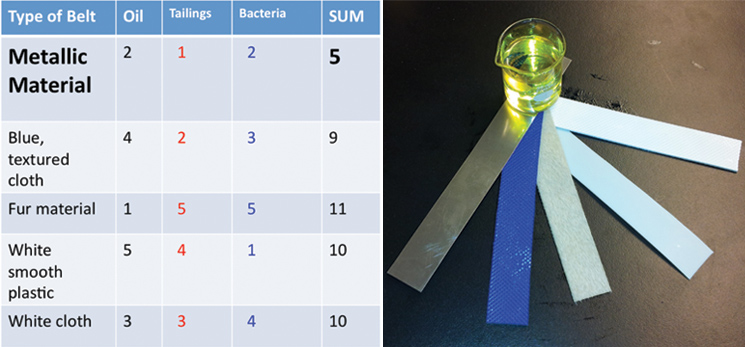
Additionally, we ran three different twenty-four hour bacterial growth tests in our tank to determine the effectiveness of the agitator and sparger on bacterial growth. The test was conducted with a turbine and a sparger, only a turbine, and only a sparger. At the end of each experiment we measured the optical density of the solution with a spectrophotometer to quantify the bacterial growth. Operating our bioreactor with both a turbine and sparger resulted in slightly greater bacterial growth than just the turbine, which coincides with our hypothesis. The results are displayed below:
Also, we mixed water, commercial NA’s, and hexadecane (model hydrocarbon) together in a small test tube to determine if we will indeed get a top layer of hydrocarbons like what we need. Indeed, this top layer of hydrocarbons was formed after two minutes of time to allow for separation.
The Final System
Next Steps
The next steps would include developing a feedback and sensing method to monitor the temperature, Ph, and C02 in a highly toxic and corrosive environment. Once the genetically engineered bacterium is produced we can start modeling the bacterial life cycle. Since our bacteria will only produce hydrocarbons within its stationary phase, we would like to look into increasing this portion of the lifecycle and possibly into adding bacteria to the bioreactor when it is in its exponential phase rather than the lag phase. This should reduce the time needed for each reactor cycle. Also, another important aspect we can test after the bacteria are made is the rate of hydrocarbon production by the bacteria.
 "
"
