Team:Stanford-Brown/HellCell/Desiccation
From 2012.igem.org
Kendrickwang (Talk | contribs) (/* Desiccation At a glance Extremophiles: Pantoea agglomerans and Saccharomyces cerevisiae Proteins of interest: choline dehydrogenase and betaine aldehyde dehydrogenase (glycine betaine biosynthesis pathway) and Osmoregulatory trehalose synthe) |
Kendrickwang (Talk | contribs) (/* Desiccation At a glance Extremophiles: Pantoea agglomerans and Saccharomyces cerevisiae Proteins of interest: choline dehydrogenase and betaine aldehyde dehydrogenase (glycine betaine biosynthesis pathway) and Osmoregulatory trehalose synthe) |
||
| Line 28: | Line 28: | ||
'''Assay''' | '''Assay''' | ||
| + | |||
Liquid cultures of negative control, recA, dps, and sdaB transformed NEB5α E. coli were grown up over night at 37oC. After the proper incubation period, a dilution spot assay was conducted on each of the cultures to determine the density of live cells. Next, 15 5cm, round petri dishes were filled with negative control bacteria, another 15 with transformants containing recA, another 15 with transformants containing dps, and another 15 with transformants containing sdaB. The petri dishes were allowed to desiccate while covered for 24 hours at 37oC while shaking. After the 24 hours period, each plate was resuspended with 1mL of fresh LB. A dilution spot assay was then conducted on each of the petri dish to determine the final density of live cells. | Liquid cultures of negative control, recA, dps, and sdaB transformed NEB5α E. coli were grown up over night at 37oC. After the proper incubation period, a dilution spot assay was conducted on each of the cultures to determine the density of live cells. Next, 15 5cm, round petri dishes were filled with negative control bacteria, another 15 with transformants containing recA, another 15 with transformants containing dps, and another 15 with transformants containing sdaB. The petri dishes were allowed to desiccate while covered for 24 hours at 37oC while shaking. After the 24 hours period, each plate was resuspended with 1mL of fresh LB. A dilution spot assay was then conducted on each of the petri dish to determine the final density of live cells. | ||
Revision as of 07:01, 3 October 2012
Desiccation
At a glance
Extremophiles: Pantoea agglomerans and Saccharomyces cerevisiae
Proteins of interest: choline dehydrogenase and betaine aldehyde dehydrogenase (glycine betaine biosynthesis pathway) and Osmoregulatory trehalose synthesis A and B (trehalose synthesis pathway)
Consensus: Effective
Saccharomyces cerevisiae, baker’s yeast, is remarkably resistant to desiccation, and it has been shown that certain osmoprotectant chemicals accumulate in these cells when desiccated. One of these chemicals is trehalose, a disaccharide that was initially implicated in desiccation resistance because of its accumulation (Calahan, Dunham, DeSevo, and Koshland 2011). Surprisingly, assays of trehalose’s effectiveness in the resistance of yeast have produced conflicting and inconsistent results (Calahan et al. 2011).
The gram-negative biocontrol agent Pantoea agglomerans was ineffective at controlling blue mold on fruits because of the long periods of dehydration that it experienced during the processing of the fruit (Bonaterra, Camps, and Montesinos 2005). Its effectiveness was significantly increased when it accumulated trehalose and glycine betaine during desiccation, and it was hypothesized that these osmoprotectants “operate through protection of membrane phospholipids by direct hydrogen bounding with phospolipid head groups maintaining the liquid crystal state and stabilising proteins by water replacement via hydrogen bounding” (Bonaterra, Camps, and Montesinos 2005). Glycine betaine was also found to have an osmoprotectant role in E. coli itself (Cayley, Lewis, and Record 1992).
To learn more about the ability of trehalose and glycine betaine and potentially apply them towards desiccation resistance, the Hell Cell Squad isolated the genes necessary for biosynthesis of trehalose and glycine betaine in E. coli and put them into the Test Plasmid. We also tested MntH mutants for desiccation resistance-conferring properties (see Radiation).
Assay
Liquid cultures of negative control, recA, dps, and sdaB transformed NEB5α E. coli were grown up over night at 37oC. After the proper incubation period, a dilution spot assay was conducted on each of the cultures to determine the density of live cells. Next, 15 5cm, round petri dishes were filled with negative control bacteria, another 15 with transformants containing recA, another 15 with transformants containing dps, and another 15 with transformants containing sdaB. The petri dishes were allowed to desiccate while covered for 24 hours at 37oC while shaking. After the 24 hours period, each plate was resuspended with 1mL of fresh LB. A dilution spot assay was then conducted on each of the petri dish to determine the final density of live cells.
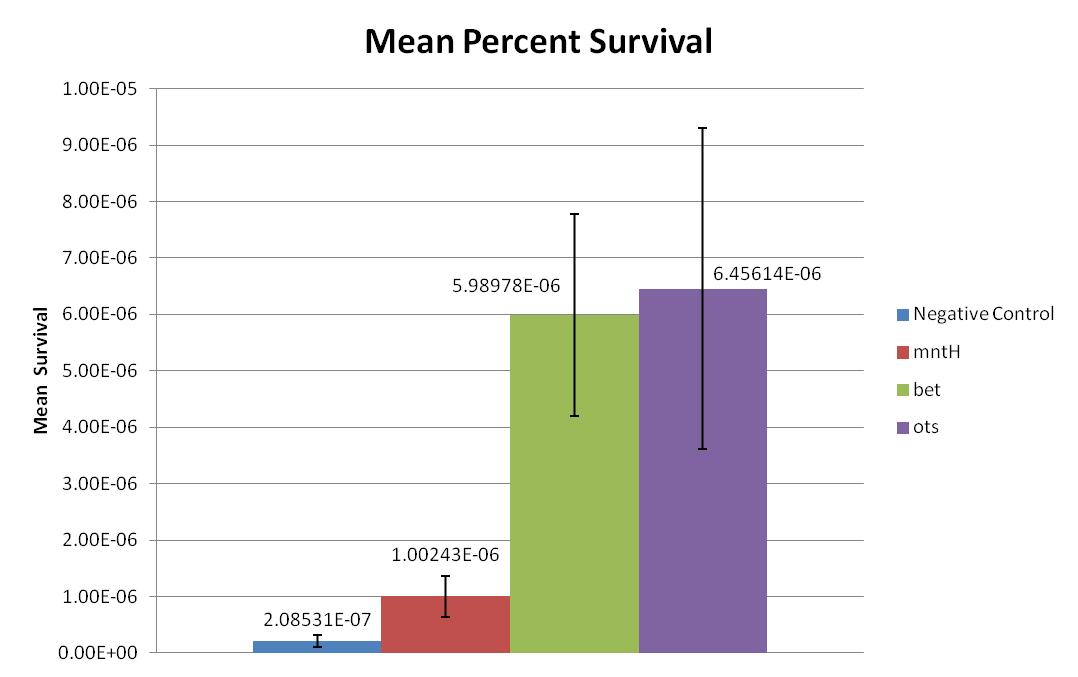
Figure 1: This graph displays the percent of surviving cells after 24 hours of desiccation. The exact survival percentages and standard errors of the mean are displayed above each sample in decimal form.
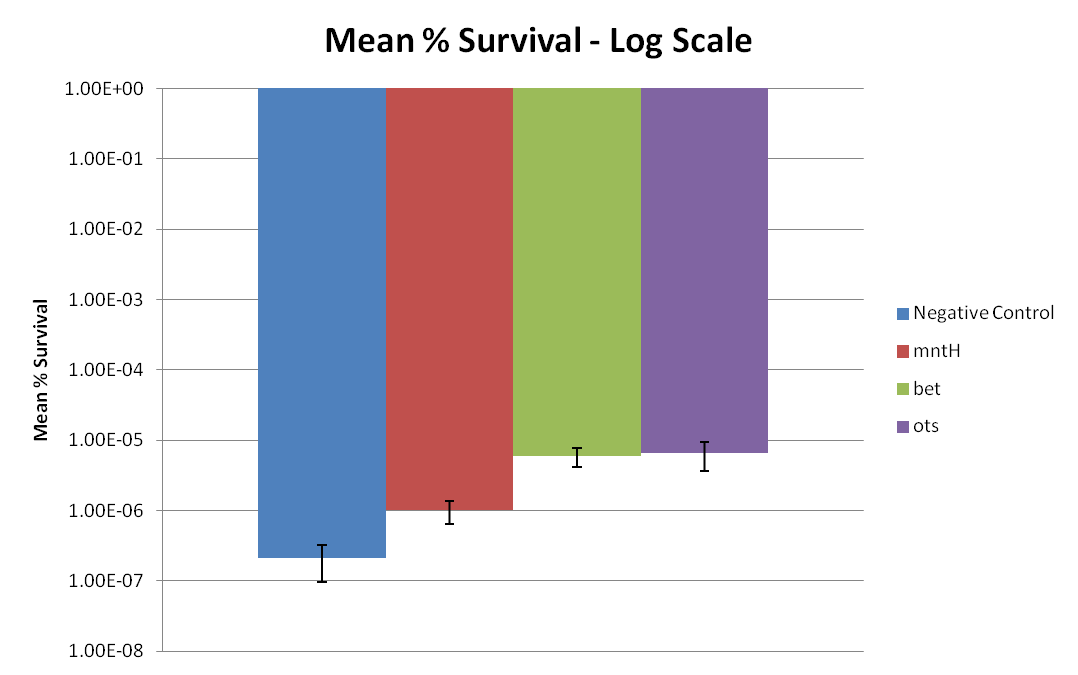
Figure 2: Displays Figure 1 in a log scale.
Conclusions
Based on cell survival after 24 hours of desiccation, it is observed that the bet construct and ots construct provides desiccation resistance. Additionally, the mntH construct may also provide slightly increased desiccation resistance. Figure 1 displays that mntH only slightly provides desiccation resistance, while bet and ots provides significantly more desiccation resistance. This trend can be more clearly observed in Figure 2. Figure 2 clearly displays that the E. coli mntH construct increases survivability by almost one order of magnitude. Additionally, Figure 2 also displays that the bet and ots constructs provide almost two orders of magnitude increase in survivability. Based on these results, it is reasonable to conclude that the bet and ots constructs provide significant desiccation resistance and that mntH provides slight desiccation resistance.
Sources:
Calahan, D., Dunham, M., DeSevo, C., Koshland, D.E. (2011). Genetic analysis of desiccation tolerance in Saccharomyces cerevisiae. Genetics, 189: 507-519.
Bonaterra, A., Camps, J., Montesinos, E. (2005). Osmotically induced trehalose and glycine betaine accumulation improves tolerance to desiccation, survival and efficacy of the postharvest biocontrol agent Pantoea agglomerans EPS125. FEMS Microbiol. Lett., 250: 1-8.
Cayley, S., Lewis, B. A., Record Jr., T. (1992). Origins of the osmoprotective properties of betaine and proline in Escherichia coli K-12. J. Bacteriol. 174(5): 1586-1595.
 "
"