Team:Tokyo Tech/Projects/positive feedback assay/index.htm
From 2012.igem.org
(→1.Construction) |
(→2.Strain) |
||
| Line 88: | Line 88: | ||
==2.Strain== | ==2.Strain== | ||
| - | JM2 | + | JM2.300 |
==3.Protocol== | ==3.Protocol== | ||
Revision as of 20:37, 26 September 2012
Materials & Methods
1.Construction
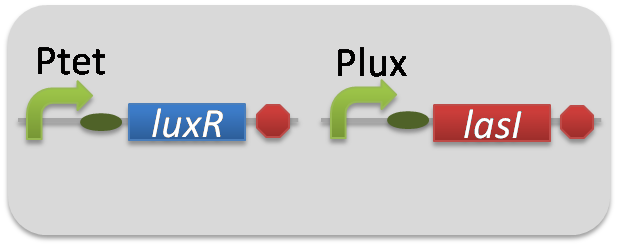
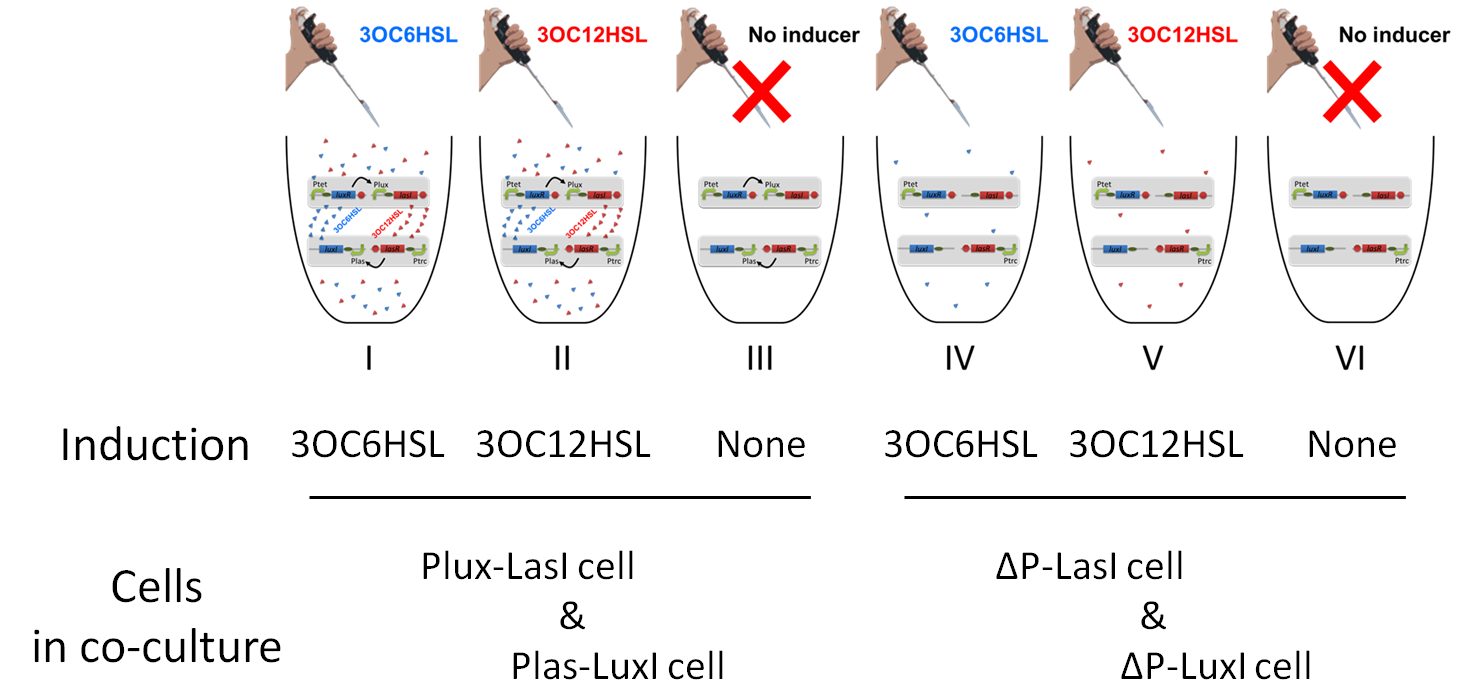
pSB6A1-Ptet-LuxR / pSB3K3-Plux-LasI (JM2.300)…Plux-LasI cell
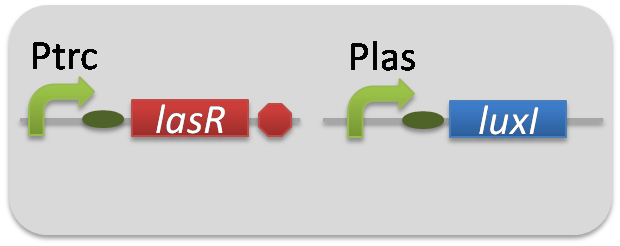
pSB6A1-Ptrc-LasR / pSB3K3-Plas-LuxI (JM2.300)…Plas-LuxI cell
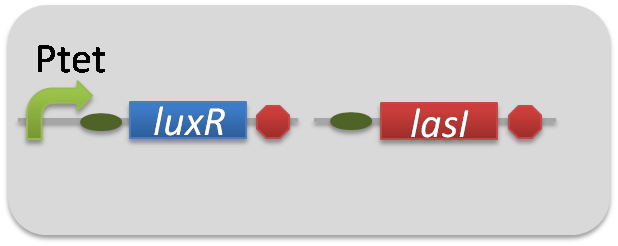
pSB6A1-Ptet-LuxR / pSB3K3-ΔP-LasI (JM2.300)…ΔP-LasI cell
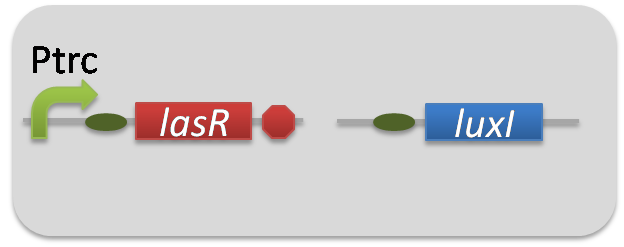
pSB6A1-Ptrc-LasR / pSB3K3-ΔP-LuxI (JM2.300)…ΔP-LuxI cell
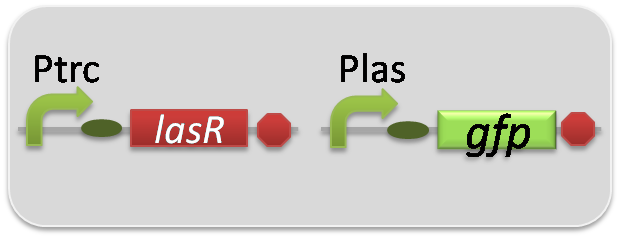
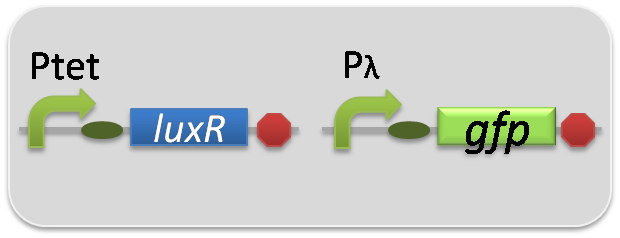
pSB6A1-Ptrc-LasR / pSB3K3-Plas-GFP (JM2.300)…Las reporter cell
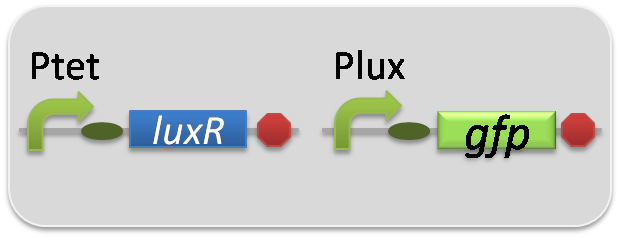
pSB6A1-Ptet-LuxR / pSB3K3-Plux-GFP (JM2.300)…Lux reporter cell
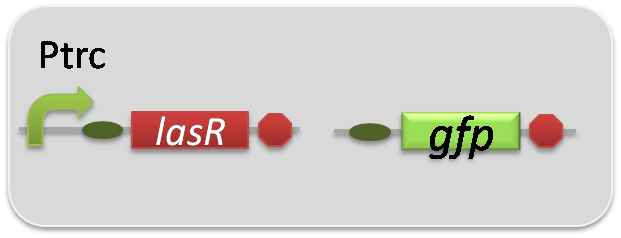
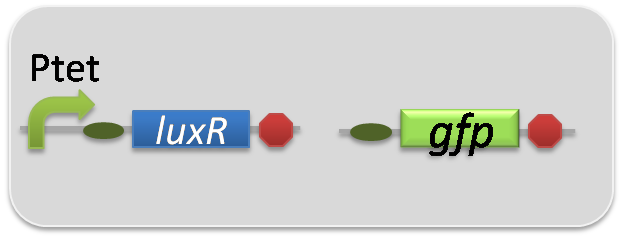
pSB6A1-Ptrc-LasR / pSB3K3-ΔP-GFP (JM2.300)…negative control
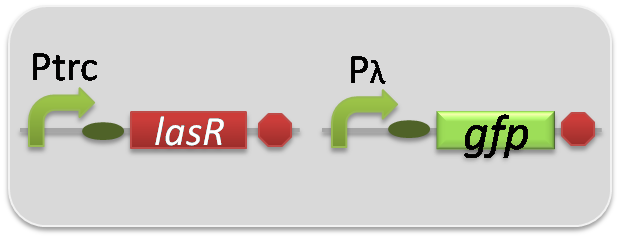
pSB6A1-Ptrc-LasR / pSB3K3-pλ-GFP (JM2.300)…positive control
pSB6A1-Ptet-LuxR / pSB3K3-ΔP-GFP (JM2.300)…negative control
pSB6A1-Ptet-LuxR / pSB3K3-pλ-GFP (JM2.300)…positive control
2.Strain
JM2.300
3.Protocol
3OC6HSL dependent
[Back to "Construction of the 3OC6HSL-dependent 3OC12HSL production module"]
1. collect liquid culture
1.1 Prepare overnight culture of inducer cell at 37°C for 12hours.
1.2 Take 30μl of the overnight culture of inducer cell into LB(3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml).(→fresh culture)
1.3 Incubate the flesh culture of inducer cell until the observed OD600 reaches around 0.50. Centrifuge the cell at 5000g, 25°C, 1 min, suspend it with 1ml LB + antibiotics (Amp 50μg/ml).
1.4 Take 30μl cell suspensions into LB(3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml) + 5μM 3OC6HSL(3μl) and LB(3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml) + DMSO(3μl).
1.5 Incubate the 3OC12HSL producer cells for another 4 hours at 37°C.
1.6 Centrifuge the 3OC12HSL producer cells at 9000g, 4°C, 1 min, and filter the cultured cells.
1.7 Dilute the filtrate by LB + antibiotics (Amp + Kan) in 1:30.
2Reporter assay
2.1 Prepare overnight culture of reporter cell at 37°C for 12hours.
2.2 Take 30μl of the overnight culture of reporter cell into LB(3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml). (→fresh culture)
2.3 Incubate the flesh culture of reporter cell until the observed OD600 reaches around 0.50, and gather the supernatant of culture of inducer cell.
2.4 Centrifuge the reporter cell at 5000g, 25°C, 1 min, and take it into LB + antibiotics (Amp 50μg/ml + Kan 30μg/ml).
2.5 Add 30μl samples of process 2.4 to filtrate + LB(3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml) from process 1.7
| 3OC6HSL | Plux |
| + | + |
| - | + |
| + | - |
| - | - |
2.6 Incubate the reporter cells for 4 hours at 37℃.
2.7 Flow cytometer measurements for GFP expression of reporter cells.
[Back to "Construction of the 3OC6HSL-dependent 3OC12HSL production module"]
3OC12HSL dependent
[Back to "Construction of the 3OC12HSL-dependent 3OC6HSL production module"]
1. collect liquid culture
1.1 Prepare overnight culture of inducer cell at 37°C for 12hours.
1.2 Take 30μl of the overnight culture of inducer cell into LB(3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml).(→fresh culture)
1.3 Incubate the flesh culture of inducer cell until the observed OD600 reaches around 0.50. Centrifuge the cell at 5000g, 25°C, 1 min, suspend it with 1ml LB + antibiotics (Amp 50μg/ml).
1.4 Take 30μl cell suspensions into LB(3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml) + 5μM 3OC6HSL(3μl) and LB(3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml) + DMSO(3μl).
1.5 Incubate the 3OC12HSL producer cells for another 4 hours at 37°C.
1.6 Centrifuge the 3OC12HSL producer cells at 9000g, 4°C, 1 min, and filter the cultured cells.
1.7 Dilute the filtrate by LB + antibiotics (Amp + Kan) in 1:30.
2 Reporter assay
2.1 Prepare overnight culture of reporter cell at 37°C for 12hours. 2.2 Take 30μl of the overnight culture of reporter cell into LB(3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml). (→fresh culture)
2.3 Incubate the flesh culture of reporter cell until the observed OD600 reaches around 0.50, and gather the supernatant of culture of inducer cell.
2.4 Centrifuge the reporter cell at 5000g, 25°C, 1 min, and take it into LB + antibiotics (Amp 50μg/ml + Kan 30μg/ml).
2.5 Add 30μl samples of process 2.4 to filtrate + LB(3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml) from process 1.7
| 3OC12HSL | Plas |
| + | + |
| - | + |
| + | - |
| - | - |
2.6 Induction of reporter cell for 4 hours at 37°C.
2.7 Flow cytometer measurements for GFP expression of reporter cell.
[Back to "Construction of the 3OC12HSL-dependent 3OC6HSL production module"]
positive feedback assay
[Back to "Construction of the positive feedback system"]
1. collect liquid culture
1.1 Prepare overnight culture of inducer cells at 37℃ for 12hours.
1.2 Take 30μl of the overnight culture of inducer cells into LB (3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml). (→fresh culture)
1.3 Incubate the flesh culture of inducer cells until the observed OD600 reaches around 0.50. Centrifuge the cells at 5000g, 25℃, 1 min, suspend it with 1ml LB + antibiotics (Amp 50μg/ml + Kan 30μg/ml).
1.4 According to the Fig.3-1-3-1-1, take 30μl cell suspensions into LB(3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml) + 3OC6HSL (5nM) or OC6HSL(2.5nM)
1.5 Incubate the inducer cell for another 4 hours at 37°C.
1.6 Centrifuge the inducer cell at 9000g, 4°C, 1 min, and filter the cultured cell.
1.7 Dilute the filtrate by LB + antibiotics (Amp 50μg/ml + Kan 30μg/ml) in 1:30.
2 Reporter assay
2.1 Prepare overnight culture of reporter cells at 37℃ for 12hours.
2.2 Take 30μl of the overnight culture of reporter cells into LB (3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml). (→fresh culture)
2.3 Incubate the flesh culture of reporter cells until the observed OD600 reaches around 0.50, and gather the supernatant of culture of inducer cells.
2.4 Centrifuge the reporter cells at 5000g, 25℃, 1 min, and take it into LB + antibiotics (Amp 50μg/ml + Kan 30μg/ml).
2.5 Add 30μl samples of process 2.4 to filtrate + LB (3ml) + antibiotics (Amp 50μg/ml + Kan 30μg/ml) from process 1.7
2.6 Incubate the reporter cells for 4 hours at 37℃.
2.7 Flow cytometer measurements for GFP expression of reporter cells.
 "
"