Team:Slovenia/Notebook
From 2012.igem.org
Experimental methods


|
| Figure 1. Schematic presentation of methods used for cloning and culturing eukaryotic cells. |
|
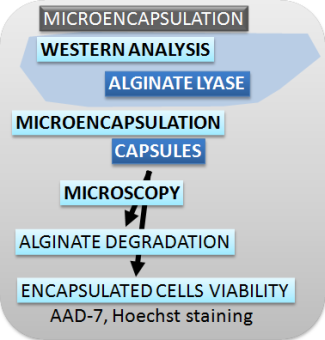
| Figure 2. Schematic presentation of methods used for characterizing the switch, safety mechanisms, microencapsulation and effectors. |
Cloning
Plasmid DNA isolation
A. MINI PREPs for analysis and sequencing- A single colony was picked from a LB-agar plate or glycerol stock and inoculated 10 mL LB-medium with appropriate antibiotic for selection (100 mg/L ampicillin, 50 mg/L kanamycin, 35 mg/L chloramphenicol).
- Bacteria were grown over night at 37 °C while shaking.
- Plasmid DNA was isolated from 2-6 mL of over-night culture with GeneJET plasmid miniprep kit according to manufacturer's protocol.
- Around 6-10 µg plasmid DNA was obtained.
- Purity and amount of DNA was analysed using a NanoDrop.
Fragment DNA isolation from agarose gel
A. AGAROSE ELECTROPHORESIS- A mixture of DNA fragments with different sizes were separated with agarose gel (0.7 and 2% agarose in 0.5x TAE buffer and 0.1 µg/ml ethidium bromide) at constant voltage of 100 V.
- UV light (λ = 254 nm) was used to visualize ethidium bromide intercalated into DNA.
- Position of DNA fragments are documented and cut out from agarose.
B. FRAGMENT ISOLATION from agarose gel
- The band with the desired DNA fragment was excised from the gel, using a clean scalpel.
- DNA was isolated from the gel slice with GeneJet Gel Extraction Kit according to manufacturers protocol.
- Purity and amount of DNA was determined using a NanoDrop.
Restriction digest
- Set up a restriction mixture:
- for analysis of cloned DNA
- 1X optimized Restriction buffer (10X)
- 0.5 µL restriction enzyme (10 U/µL)
- Bring volume to 20 µL with nuclease-free water.
- for isolation of specific DNA
- 1X optimized Restriction buffer 10X
- up to 2 µL restriction enzyme 10 U/µL
- Bring volume to 50 µL with nuclease-free water.
- The sample was incubated at optimal temperature for the restriction enzyme(s)
- Analysis of fragmented DNA was done by gel electrophoreses (loading dye was added to the samples before loaded on a agarose gel)
- Results were documented with
- Desired DNA fragment was excised and purified using suitable DNA purification kit.
PCR
A. PCR REACTIONAccuPrime and Phusion polymerase were used for DNA amplification. Colony PCR was preformed with Taq polymerase.
- For Phusion polymerase: master mix contained
- DNA (1-10 ng)
- both primers (0,4 pmol/µl )
- 1x Phusion HF buffer
- 0,2 µM dNTPs
- Phusion polymerase (0,02 U/ µl) and
- MQ up to final volume of 25 µl
-
vAccuPrime reaction contained:
- DNA (10 ng),
- both primers (0,4 pmol/µl ),
- 1xRnx mix,
- enzyme (0,05 U/ µl) and
- MQ up to final volume of 50 µl.
| US | CAN | FR | GER | IT | SP | UK | JP | AUS | MEX | |
|---|---|---|---|---|---|---|---|---|---|---|
| Biologic sales, exmanufacturer ($US millions) | $34,957 | $1,142 | $3,828 | $3,736 | $2,106 | $2,009 | $1,864 | $5,051 | $553 | $65 |
Next: Lablog >>
 "
"