Team:Slovenia/Implementation
From 2012.igem.org
Miha Jerala (Talk | contribs) |
|||
| (11 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
<head> | <head> | ||
<meta http-equiv="X-UA-Compatible" content="IE=edge" /> | <meta http-equiv="X-UA-Compatible" content="IE=edge" /> | ||
| + | <!-- back to top --> | ||
| + | <div style="position:fixed; bottom:45px; right:30px; width:100px; height:66px; background-color:transparent;"> | ||
| + | <a href="#topofthepage"> | ||
| + | <table style="background-color:transparent;" onclick="window.location = '#topofthepage'" class="invisible" style="height:100%;"> | ||
| + | <tr class="invisible" style="background-color:transparent;"> | ||
| + | <td class="invisible" style="background-color:transparent;" valign="center"> | ||
| + | <img width="100px" src ="https://static.igem.org/mediawiki/2012/1/14/Svn12_hp_btt.png"></img> | ||
| + | </td></tr></table> | ||
| + | </a> | ||
| + | </div> | ||
| + | |||
<style type="text/css"> | <style type="text/css"> | ||
| Line 201: | Line 212: | ||
#cssmenu ul li > ul li{display:block; list-style:inside none; padding:0; margin:0; position:relative;} | #cssmenu ul li > ul li{display:block; list-style:inside none; padding:0; margin:0; position:relative;} | ||
#cssmenu ul li > ul li a{ outline:none; display:block; position:relative; margin:0; padding:8px 20px; font:10pt Arial, Helvetica, sans-serif; color:#fff; text-decoration:none; text-shadow:1px 1px 0 rgba(0,0,0, 0.5); } | #cssmenu ul li > ul li a{ outline:none; display:block; position:relative; margin:0; padding:8px 20px; font:10pt Arial, Helvetica, sans-serif; color:#fff; text-decoration:none; text-shadow:1px 1px 0 rgba(0,0,0, 0.5); } | ||
| + | #cssmenu ul li > ul li a table tr td span{ outline:none; display:block; position:relative; margin:0; padding:0px 0px; font:10pt Arial, Helvetica, sans-serif; color:#fff; text-decoration:none; text-shadow:1px 1px 0 rgba(0,0,0, 0.5); } | ||
#cssmenu, #cssmenu > ul > li > ul > li a:hover | #cssmenu, #cssmenu > ul > li > ul > li a:hover | ||
{ background:#043A6B; | { background:#043A6B; | ||
| Line 217: | Line 229: | ||
/* end CSS navigation menu (blue) */ | /* end CSS navigation menu (blue) */ | ||
| + | /*new table start*/ | ||
| + | table.newtable {background-color:transparent;} | ||
| + | td.newtable, th.newtable {background-color:transparent;} | ||
| + | thead.newtable{ } | ||
| + | tbody .newtable{} | ||
| + | /*new table start*/ | ||
ul { | ul { | ||
| Line 292: | Line 310: | ||
<body> | <body> | ||
| + | <a name="topofthepage" style="background-color:transparent;"></a> | ||
<div id="banner"> | <div id="banner"> | ||
| - | |||
</div> | </div> | ||
| Line 316: | Line 334: | ||
<li><a href='https://2012.igem.org/Team:Slovenia/TheSwitchDesignedTALregulators'><span>Designed TAL regulators</span></a></li> | <li><a href='https://2012.igem.org/Team:Slovenia/TheSwitchDesignedTALregulators'><span>Designed TAL regulators</span></a></li> | ||
<li><a href='https://2012.igem.org/Team:Slovenia/TheSwitchMutualRepressorSwitch'><span>Mutual repressor switch</span></a></li> | <li><a href='https://2012.igem.org/Team:Slovenia/TheSwitchMutualRepressorSwitch'><span>Mutual repressor switch</span></a></li> | ||
| - | <li><a href='https://2012.igem.org/Team:Slovenia/TheSwitchPositiveFeedbackLoopSwitch'><span>Positive feedback loop switch</span></a></li> | + | <li><a href='https://2012.igem.org/Team:Slovenia/TheSwitchPositiveFeedbackLoopSwitch'><table onclick="window.location = 'https://2012.igem.org/Team:Slovenia/TheSwitchPositiveFeedbackLoopSwitch';" class="newtable"><tr class="newtable"><td class="newtable"><span>Positive feedback loop switch</span></td><td class="newtable"><img style="margin-right:-15px;" width="25px" src="https://static.igem.org/mediawiki/2012/e/ee/Svn12_hp_new.png"></img></td></tr></table></a></li> |
| + | <li><a href='https://2012.igem.org/Team:Slovenia/TheSwitchControls'><table onclick="window.location = 'https://2012.igem.org/Team:Slovenia/TheSwitchControls';" class="newtable"><tr class="newtable"><td class="newtable"><span>Controls</span></td><td class="newtable"><img style="margin-right:-81px;" width="25px" src="https://static.igem.org/mediawiki/2012/e/ee/Svn12_hp_new.png"></img></td></tr></table></a></li> | ||
</ul> | </ul> | ||
</li> | </li> | ||
| Line 324: | Line 343: | ||
<li><a href='https://2012.igem.org/Team:Slovenia/SafetyMechanismsEscapeTag'><span>Escape tag</span></a></li> | <li><a href='https://2012.igem.org/Team:Slovenia/SafetyMechanismsEscapeTag'><span>Escape tag</span></a></li> | ||
<li><a href='https://2012.igem.org/Team:Slovenia/SafetyMechanismsTermination'><span>Termination</span></a></li> | <li><a href='https://2012.igem.org/Team:Slovenia/SafetyMechanismsTermination'><span>Termination</span></a></li> | ||
| - | + | <li><a href="https://2012.igem.org/Team:Slovenia/SafetyMechanismsMicrocapsuleDegradation"><table onclick="window.location = 'https://2012.igem.org/Team:Slovenia/SafetyMechanismsMicrocapsuleDegradation';" class="newtable"><tr class="newtable"><td class="newtable"><span>Microcapsule degradation</span></td><td class="newtable"><img style="margin-right:-15px;" width="25px" src="https://static.igem.org/mediawiki/2012/e/ee/Svn12_hp_new.png"></img></td></tr></table></a></li> | |
</ul> | </ul> | ||
</li> | </li> | ||
| Line 332: | Line 351: | ||
<li><a href='https://2012.igem.org/Team:Slovenia/ImplementationHepatitisC'><span>Hepatitis C</span></a></li> | <li><a href='https://2012.igem.org/Team:Slovenia/ImplementationHepatitisC'><span>Hepatitis C</span></a></li> | ||
<li><a href='https://2012.igem.org/Team:Slovenia/ImplementationIschaemicHeartDisease'><span>Ischaemic heart disease</span></a></li> | <li><a href='https://2012.igem.org/Team:Slovenia/ImplementationIschaemicHeartDisease'><span>Ischaemic heart disease</span></a></li> | ||
| + | <li><a href='https://2012.igem.org/Team:Slovenia/ImplementationImpact'><table onclick="window.location = 'https://2012.igem.org/Team:Slovenia/ImplementationImpact';" class="newtable"><tr class="newtable"><td class="newtable"><span>Impact</span></td><td class="newtable"><img style="margin-right:-86px;" width="25px" src="https://static.igem.org/mediawiki/2012/e/ee/Svn12_hp_new.png"></img></td></tr></table></a></li> | ||
</ul> | </ul> | ||
| Line 339: | Line 359: | ||
<ul> | <ul> | ||
<li><a href='https://2012.igem.org/Team:Slovenia/Modeling'><span>Overview</span></a></li> | <li><a href='https://2012.igem.org/Team:Slovenia/Modeling'><span>Overview</span></a></li> | ||
| - | + | <li><a href='https://2012.igem.org/Team:Slovenia/ModelingPK'><table onclick="window.location = 'https://2012.igem.org/Team:Slovenia/ModelingPK';" class="newtable"><tr class="newtable"><td class="newtable"><span>Pharmacokinetics</span></td><td class="newtable"><img style="margin-right:-15px;" width="25px" src="https://static.igem.org/mediawiki/2012/e/ee/Svn12_hp_new.png"></img></td></tr></table></a></li> | |
<li><a href='https://2012.igem.org/Team:Slovenia/ModelingMethods'><span>Modeling methods</span></a></li> | <li><a href='https://2012.igem.org/Team:Slovenia/ModelingMethods'><span>Modeling methods</span></a></li> | ||
<li><a href='https://2012.igem.org/Team:Slovenia/ModelingMutualRepressorSwitch'><span>Mutual repressor switch</span></a></li> | <li><a href='https://2012.igem.org/Team:Slovenia/ModelingMutualRepressorSwitch'><span>Mutual repressor switch</span></a></li> | ||
<li><a href='https://2012.igem.org/Team:Slovenia/ModelingPositiveFeedbackLoopSwitch'><span>Positive feedback loop switch</span></a></li> | <li><a href='https://2012.igem.org/Team:Slovenia/ModelingPositiveFeedbackLoopSwitch'><span>Positive feedback loop switch</span></a></li> | ||
| - | <li><a href='https://2012.igem.org/Team:Slovenia/ModelingQuantitativeModel'><span> | + | <li><a href='https://2012.igem.org/Team:Slovenia/ModelingQuantitativeModel'><table onclick="window.location = 'https://2012.igem.org/Team:Slovenia/ModelingQuantitativeModel';" class="newtable"><tr class="newtable"><td class="newtable"><span>Experimental model</span></td><td class="newtable"><img style="margin-right:-15px;" width="25px" src="https://static.igem.org/mediawiki/2012/e/ee/Svn12_hp_new.png"></img></td></tr></table></a></li> |
| - | + | <li><a href='https://2012.igem.org/Team:Slovenia/ModelingInteractiveSimulations'><table onclick="window.location = 'https://2012.igem.org/Team:Slovenia/ModelingInteractiveSimulations';" class="newtable"><tr class="newtable"><td class="newtable"><span>Interactive simulations</span></td><td class="newtable"><img style="margin-right:-15px;" width="25px" src="https://static.igem.org/mediawiki/2012/e/ee/Svn12_hp_new.png"></img></td></tr></table></a></li> | |
</ul> | </ul> | ||
</li> | </li> | ||
| Line 355: | Line 375: | ||
<ul> | <ul> | ||
<li><a href='https://2012.igem.org/Team:Slovenia/Notebook'><span>Experimental methods</span></a></li> | <li><a href='https://2012.igem.org/Team:Slovenia/Notebook'><span>Experimental methods</span></a></li> | ||
| - | + | <li><a href='https://2012.igem.org/Team:Slovenia/NotebookLablog'><table onclick="window.location = 'https://2012.igem.org/Team:Slovenia/NotebookLablog';" class="newtable"><tr class="newtable"><td class="newtable"><span>Lablog</span></td><td class="newtable"><img style="margin-right:-90px;" width="25px" src="https://static.igem.org/mediawiki/2012/e/ee/Svn12_hp_new.png"></img></td></tr></table></a></li> | |
<li><a href='https://2012.igem.org/Team:Slovenia/NotebookLabSafety'><span>Lab safety</span></a></li> | <li><a href='https://2012.igem.org/Team:Slovenia/NotebookLabSafety'><span>Lab safety</span></a></li> | ||
</ul> | </ul> | ||
| Line 378: | Line 398: | ||
<li><a href='https://2012.igem.org/Team:Slovenia/Team'><span>Team members</span></a></li> | <li><a href='https://2012.igem.org/Team:Slovenia/Team'><span>Team members</span></a></li> | ||
<li><a href='https://2012.igem.org/Team:Slovenia/TeamAttributions'><span>Attributions</span></a></li> | <li><a href='https://2012.igem.org/Team:Slovenia/TeamAttributions'><span>Attributions</span></a></li> | ||
| + | <li><a href='https://2012.igem.org/Team:Slovenia/TeamCollaborations'><table onclick="window.location = 'https://2012.igem.org/Team:Slovenia/TeamCollaborations';" class="newtable"><tr class="newtable"><td class="newtable"><span>Collaborations</span></td><td class="newtable"><img style="margin-right:-20px;" width="25px" src="https://static.igem.org/mediawiki/2012/e/ee/Svn12_hp_new.png"></img></td></tr></table></a></li> | ||
<li><a href='https://2012.igem.org/Team:Slovenia/TeamGallery'><span>Gallery</span></a></li> | <li><a href='https://2012.igem.org/Team:Slovenia/TeamGallery'><span>Gallery</span></a></li> | ||
<li><a href='https://2012.igem.org/Team:Slovenia/TeamSponsors'><span>Sponsors</span></a></li> | <li><a href='https://2012.igem.org/Team:Slovenia/TeamSponsors'><span>Sponsors</span></a></li> | ||
| Line 390: | Line 411: | ||
<div id="main"> | <div id="main"> | ||
<br/> | <br/> | ||
| + | |||
| + | <!-- dummy link na bannerju --> | ||
| + | <a href="https://2012.igem.org/Main_Page"> | ||
| + | <div id="dummy" style="background-color:transparent; position:absolute; left:870px; top:25px; width:115px; height:80px; z-index:100; opacity:0.0;"> | ||
| + | </div> | ||
| + | </a> | ||
| + | |||
<!-- VSEBINA OD TU DALJE --> | <!-- VSEBINA OD TU DALJE --> | ||
| - | <h1> | + | <h1>Implementation overview</h1> |
| - | <p>We have considered | + | |
| - | <ul style=" | + | <table class="summary"> |
| - | <li>The therapy of hepatitis C by the local production of interferon alpha followed by the production of hepatocyte growth factor, | + | <tr class="summary"> |
| - | <li>The therapy of ischaemic heart disease with anakinra as an | + | <td class="summary"> |
| + | |||
| + | |||
| + | <p>Ideas for the therapeutic implementation were contributed by the medical student team members and were verified and refined through consultation with medical experts from the University clinical centre of Ljubljana, who proposed technical solutions for clinical implementations. We used therapeutics whose efficiency has already been demonstrated either in the clinics or at least in animal models.</p> | ||
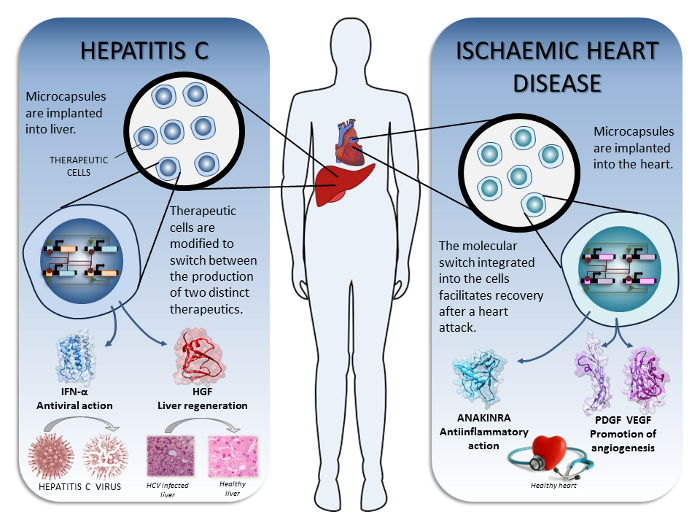
| + | <p>In our iGEM project we have considered implementing therapy with engineered cells able to perstist in and switch between two defined states, but our orthogonal switch allows many more advanced combinations. For the demonstration of the proof of principle we implemented the switch device for two diseases that could be treated by the use of our synthetic microencapsulated cellular device (Figure 1) and analyzed the pharmacokinetics of this type of drug delivery: </p> | ||
| + | |||
| + | |||
| + | <ul style="margin-left:30px;"> | ||
| + | <li>The therapy of <b>hepatitis C</b> by the local production of <a href=”http://partsregistry.org/wiki/index.php?title=Part:BBa_K782060”>interferon alpha</a> followed by the production of hepatocyte growth factor, promoting liver regeneration.</li> | ||
| + | <li>The therapy of <b>ischaemic heart disease</b> with anakinra as an anti-inflammatory agent, followed by <a href=”http://partsregistry.org/wiki/index.php?title=Part:BBa_K782061”>vascular endothelial growth factor and platelet-derived growth factor</a>, whose production in stoichiometric amounts enhances angiogenesis and tissue oxygenation and prevents the immature blood vessel formation effects of using only VEGF. </li> | ||
</ul> | </ul> | ||
| + | |||
| + | <br /> | ||
| + | <p>Clearly all therapeutic regimens will have to first be tested in preclinical studies to identify the optimal therapeutic combinations and doses.</p> | ||
| + | |||
| + | |||
| + | |||
<br/> | <br/> | ||
| - | <img src="https://static.igem.org/mediawiki/2012/b/b3/Svn12_implementation_overview_fig1.png"></ | + | </td> |
| - | < | + | </tr> |
| + | </table> | ||
| + | |||
| + | <p> | ||
| + | <!-- figure 1 --> | ||
| + | <table class="invisible" style="width:90%;"> | ||
| + | <tbody class="invisible"> | ||
| + | <tr class="invisible"> | ||
| + | <td class="invisible"> | ||
| + | <img class="invisible" src="https://static.igem.org/mediawiki/2012/b/b3/Svn12_implementation_overview_fig1.png"/> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </tbody> | ||
| + | </table> | ||
| + | <table class="invisible" style="width:90%; text-align:center;"> | ||
| + | <tbody class="invisible"> | ||
| + | <tr class="normal"><td class="invisible"> | ||
| + | <b>Figure 1. The proposed therapeutic implementations of our synthetic biology device.</b> | ||
| + | </td></tr> | ||
| + | </tbody> | ||
| + | </table> | ||
| + | <!-- end table--> | ||
| + | </p> | ||
| + | |||
</br> | </br> | ||
<h3>Important advantages, considerations and limitations of the therapeutic implementation of the synthetic cellular devices based on cell microencapsulation</h3> | <h3>Important advantages, considerations and limitations of the therapeutic implementation of the synthetic cellular devices based on cell microencapsulation</h3> | ||
<h4>Advantages</h4> | <h4>Advantages</h4> | ||
| + | |||
<ul style="padding-left:30px"> | <ul style="padding-left:30px"> | ||
| - | <li>The ability to produce several protein therapeutics at the same time or <b>in a defined temporal sequence | + | <li>The ability to produce several protein therapeutics at the same time or <b>in a defined temporal sequence</b>.</li> |
<ul style="padding-left:60px"> | <ul style="padding-left:60px"> | ||
<li>Two therapeutics under the control of different operators can be timed to expression at different stages of the disease or its therapy.</li> | <li>Two therapeutics under the control of different operators can be timed to expression at different stages of the disease or its therapy.</li> | ||
| - | <li>Introduction of the p2a (or t2a) amino acid sequence in frame between two or more protein coding sequences allows concurrent stoichiometric production of these proteins (Szymczak et al., 2004; see stimulation of angiogenesis in the therapy of | + | <li>Introduction of the p2a (or t2a) amino acid sequence in frame between two or more protein coding sequences allows concurrent stoichiometric production of these proteins (Szymczak et al., 2004; see stimulation of angiogenesis in the therapy of ischaemia for an example, Banfi et al., 2012). </li> |
</ul> | </ul> | ||
| - | <li>The ability to <b>switch between several therapeutic regimens | + | <li>The ability to <b>switch between several therapeutic regimens</b>.</li> |
| - | <li>Therapeutic action can be | + | <li>Therapeutic action can be localized within the affected tissue.</li> |
| - | <li>Engineered therapeutic cells can mimic stem cells, which secrete many growth factors (e.g. the antiinflammatory and antifibrotic effect of | + | <li>Engineered therapeutic cells can mimic stem cells, which secrete many growth factors (e.g. the antiinflammatory and antifibrotic effect of mesenchymal stem cells in lung injury was attributed to secretion of IL-1R antagonist (Ortiz et al., 2007).</li> |
| - | <li>Continuous production of a therapeutic protein, <b>avoiding peaks and | + | <li>Continuous production of a therapeutic protein, <b>avoiding peaks and lows in drug concentration, a reduction in the side effects</b> and <b>an increase in the duration of an effective concentration within the target tissue.</b> |
</ul> | </ul> | ||
| + | |||
| + | |||
| + | |||
<h4>Limitations</h4> | <h4>Limitations</h4> | ||
<ul style="padding-left:30px"> | <ul style="padding-left:30px"> | ||
| - | <li>The efficient exchange of nutrients is limited by the size of the microcapsules therefore posing limitations to the maximal | + | <li>The efficient exchange of nutrients is limited by the size of the microcapsules therefore posing limitations to the maximal amount of encapsulated cells.</i> |
| - | <li>The amount of protein production is limited by the number of microcapsules, the number of encapsulated cells per | + | <li>The amount of protein production is limited by the number of microcapsules, the number of encapsulated cells per microcapsule and the maximum productivity of each cell.</li> |
<li>The choice of therapeutics is mainly limited to proteins, such as hormones, growth factors, enzymes, cytokines or antibodies.</li> | <li>The choice of therapeutics is mainly limited to proteins, such as hormones, growth factors, enzymes, cytokines or antibodies.</li> | ||
<li>Productivity of microencapsulated cells may vary with time.</li> | <li>Productivity of microencapsulated cells may vary with time.</li> | ||
| + | |||
</ul> | </ul> | ||
| + | |||
| + | |||
| + | |||
| + | |||
<h4>Other considerations for specific therapies</h4> | <h4>Other considerations for specific therapies</h4> | ||
<ul style="padding-left:30px"> | <ul style="padding-left:30px"> | ||
<li>The site of microcapsule implantation.</li> | <li>The site of microcapsule implantation.</li> | ||
<ul style="padding-left:60px"> | <ul style="padding-left:60px"> | ||
| - | <li>Does the therapeutic protein readily | + | <li>Does the therapeutic protein readily cross microcapsules and penetrate into the diseased tissue?</li> |
<li>Is implantation safe and anatomically feasible?</li> | <li>Is implantation safe and anatomically feasible?</li> | ||
<li>Would it be too invasive to implant microcapsules?</li> | <li>Would it be too invasive to implant microcapsules?</li> | ||
</ul> | </ul> | ||
| - | <li>How many cells are required to produce sufficient therapeutic amounts of the biological drug? We estimate that therapeutic production by encapsulated cells can probably reach up to a few tens of µg per day. This suggests most perspective therapeutics would be hormones, enzymes, cytokines and other proteins that act as regulators or are catalytic, while neutralizers such as antibodies are appropriate only for highly localized production.</li> | + | <li>How many cells are required to produce sufficient therapeutic amounts of the biological drug? We estimate that therapeutic production by encapsulated cells can probably reach up to a few tens of µg per day. This suggests that the most perspective therapeutics would be hormones, enzymes, cytokines and other proteins that act as regulators or are catalytic, while neutralizers such as antibodies are appropriate only for highly localized production.</li> |
<li>Microencapsulated synthetic cellular devices are expected to be best suited for therapy of acute disorders with duration of the therapy up to a few weeks or months rather than for chronic diseases. </li> | <li>Microencapsulated synthetic cellular devices are expected to be best suited for therapy of acute disorders with duration of the therapy up to a few weeks or months rather than for chronic diseases. </li> | ||
| + | |||
</ul> | </ul> | ||
</br> | </br> | ||
| Line 456: | Line 532: | ||
</body> | </body> | ||
| + | |||
</html> | </html> | ||
Latest revision as of 20:44, 26 October 2012
Implementation overview
|
Ideas for the therapeutic implementation were contributed by the medical student team members and were verified and refined through consultation with medical experts from the University clinical centre of Ljubljana, who proposed technical solutions for clinical implementations. We used therapeutics whose efficiency has already been demonstrated either in the clinics or at least in animal models. In our iGEM project we have considered implementing therapy with engineered cells able to perstist in and switch between two defined states, but our orthogonal switch allows many more advanced combinations. For the demonstration of the proof of principle we implemented the switch device for two diseases that could be treated by the use of our synthetic microencapsulated cellular device (Figure 1) and analyzed the pharmacokinetics of this type of drug delivery:
Clearly all therapeutic regimens will have to first be tested in preclinical studies to identify the optimal therapeutic combinations and doses. |
|
| Figure 1. The proposed therapeutic implementations of our synthetic biology device. |
Important advantages, considerations and limitations of the therapeutic implementation of the synthetic cellular devices based on cell microencapsulation
Advantages
- The ability to produce several protein therapeutics at the same time or in a defined temporal sequence.
- Two therapeutics under the control of different operators can be timed to expression at different stages of the disease or its therapy.
- Introduction of the p2a (or t2a) amino acid sequence in frame between two or more protein coding sequences allows concurrent stoichiometric production of these proteins (Szymczak et al., 2004; see stimulation of angiogenesis in the therapy of ischaemia for an example, Banfi et al., 2012).
- The ability to switch between several therapeutic regimens.
- Therapeutic action can be localized within the affected tissue.
- Engineered therapeutic cells can mimic stem cells, which secrete many growth factors (e.g. the antiinflammatory and antifibrotic effect of mesenchymal stem cells in lung injury was attributed to secretion of IL-1R antagonist (Ortiz et al., 2007).
- Continuous production of a therapeutic protein, avoiding peaks and lows in drug concentration, a reduction in the side effects and an increase in the duration of an effective concentration within the target tissue.
Limitations
- The efficient exchange of nutrients is limited by the size of the microcapsules therefore posing limitations to the maximal amount of encapsulated cells.
- The amount of protein production is limited by the number of microcapsules, the number of encapsulated cells per microcapsule and the maximum productivity of each cell.
- The choice of therapeutics is mainly limited to proteins, such as hormones, growth factors, enzymes, cytokines or antibodies.
- Productivity of microencapsulated cells may vary with time.
Other considerations for specific therapies
- The site of microcapsule implantation.
- Does the therapeutic protein readily cross microcapsules and penetrate into the diseased tissue?
- Is implantation safe and anatomically feasible?
- Would it be too invasive to implant microcapsules?
- How many cells are required to produce sufficient therapeutic amounts of the biological drug? We estimate that therapeutic production by encapsulated cells can probably reach up to a few tens of µg per day. This suggests that the most perspective therapeutics would be hormones, enzymes, cytokines and other proteins that act as regulators or are catalytic, while neutralizers such as antibodies are appropriate only for highly localized production.
- Microencapsulated synthetic cellular devices are expected to be best suited for therapy of acute disorders with duration of the therapy up to a few weeks or months rather than for chronic diseases.
References
Banfi, A., von Degenfeld, G., Gianni-Barrera, R., Reginato, S., Merchant, M.J., McDonald, D.M., and Blau, H.M. (2012) Therapeutic angiogenesis due to balanced single-vector delivery of VEGF and PDGF-BB. FASEB J. 26, 2486-2497.
Ortiz, L.A., Dutreil, M., Fattman, C., Pandey, A.C., Torres, G., Go, K., and Phinney, D.G. (2007) Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc. Natl. Acad. Sci. U S A 104, 11002-11007.
Szymczak, A.L., Workman, C.J., Wang, Y., Vignali, K.M., Dilioglou, S., Vanin, E.F., Vignali D.A. (2004) Correction of multi-gene deficiency in vivo using a single 'self-cleaving' 2A peptide-based retroviral vector. Nat. Biotechnol. 22, 589-94.
Next: Hepatitis C >>
 "
"

