Team:SJTU-BioX-Shanghai/Project/project1.3
From 2012.igem.org
(→Background) |
AleAlejandro (Talk | contribs) (→RNA Signal) |
||
| (76 intermediate revisions not shown) | |||
| Line 25: | Line 25: | ||
__NOTOC__ | __NOTOC__ | ||
<!----------------------------------------------------从这里开始写wiki---------------------------------> | <!----------------------------------------------------从这里开始写wiki---------------------------------> | ||
| - | + | =Membrane Rudder= | |
| + | {{Template:12SJTU_part_summary_head}} | ||
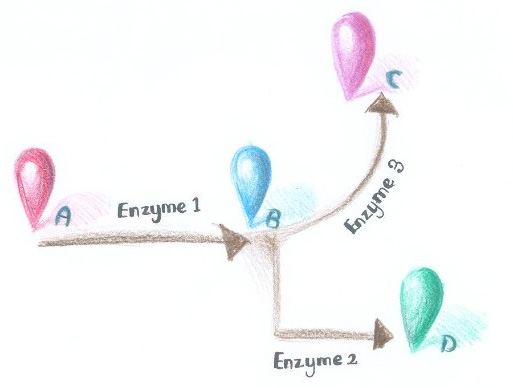
| - | + | Dynamically and artificially regulating the direction of biochemical pathway ''in vivo'' has remained a challenge for scientists. We were trying to achieve this goal through controlling the aggregation state of different enzymes. If we replace certain constitutively dimerizing proteins in ''Membrane Accelerator'' with signal-induced dimers, then it is possible to dynamically control the direction of branched reactions. In this way, a ''Membrane Rudder'' to regulate the direction of biochemical pathway could be created. | |
| - | [[Image:12SJTU_divergentreaction.jpg|thumb| | + | [[Image:12SJTU_divergentreaction.jpg|thumb|400px|right|''Fig.1'' :Demonstration of branched reactions.]] |
| + | Here is a simplified example. As is described in Figure 1, when signal that induces the aggregation of Enzyme 1 and 2 is present, the two enzymes would get close to each other, making product D more dominant. On the contrary, when signal that induces the aggregation of Enzyme 1 and 3 is present, Enzyme 1 and 3 get close to each other, so product C is more dominant. | ||
| - | + | There are indeed many signal-induced dimers that commonly exist in nature, which could sense light, chemical, peptide and even RNA signal. | |
| - | + | {{Template:12SJTU_part_summary_foot}} | |
| - | + | ==Potential Signal Pool== | |
| + | ===Light Signal=== | ||
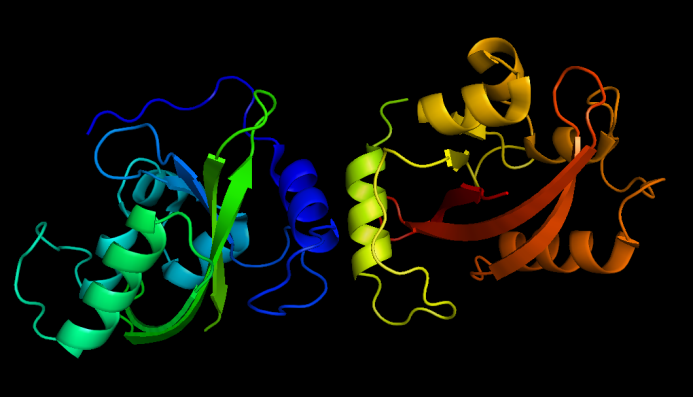
| + | [[Image:VVD dark state.png|thumb|320px|left|''Fig.2'' :Dark state VVD dimers, from PDB ID 2PD7]] | ||
| + | Light is an intriguing signal to regulate ''E.coli'' activity because it is easy to obtain, highly tunable and nontoxic. A metabolite-coupled light-switchable system could be quite fascinating. | ||
| + | |||
| + | Vivid(VVD) protein, a photoreceptor of ''Neurospora crassa'' can form dimer in the presence of blue light and disassociate as light is off. Besides, VVD protein belongs to the Per-Arnt-Sim(PAS) protein superfamily. | ||
| + | |||
| + | Compared with wildtype VVD, VVD mutant (C71V and N56K), [http://partsregistry.org/Part:BBa_K771109 Part:BBa_K771109], is harder to dimerize in the dark and easier to dimerize under blue light . So this mutant is ideal as signal sensor in ''Membrane Rudder'' device. Membrane Anchors combined with VVD are [http://partsregistry.org/Part:BBa_K771005 Part:BBa_K771005] and [http://partsregistry.org/Part:BBa_K771006 Part:BBa_K771006], named Membrane Anchor 5 and 6 respectively. | ||
| + | |||
| + | Whether blue light is present or not could determine which enzymes should be assembled to make their corresponding product dominate. | ||
| + | |||
| + | ===RNA Signal=== | ||
| + | |||
| + | |||
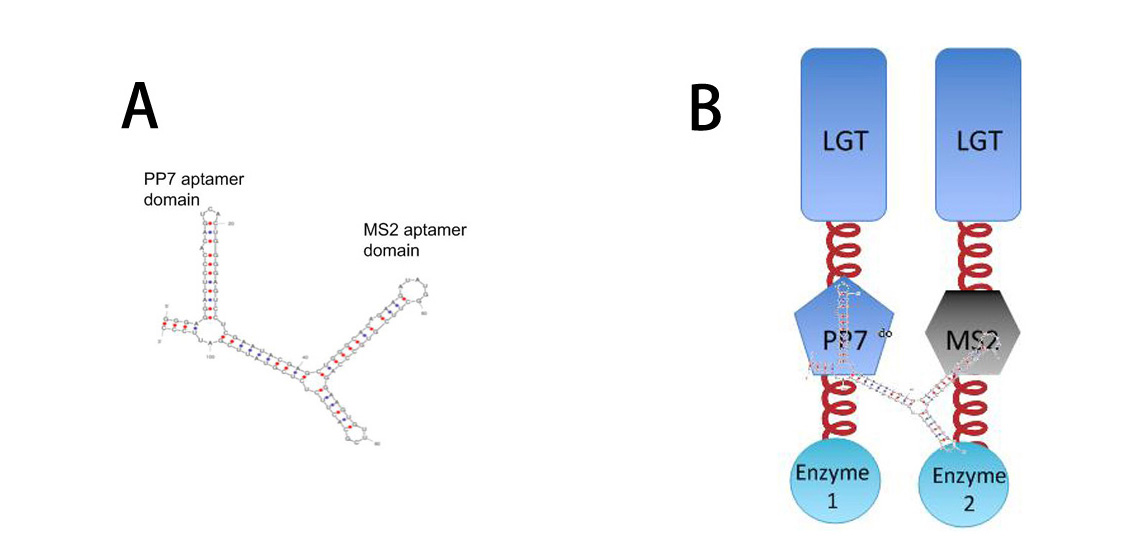
| + | So far, ''Membrane Accelerator'' and ''Membrane Rudder'' are both post-translational controlling device to regulate metabolic flux of the host cell. To connect this relatively isolated post-translational control system to genetic circuits, we employed RNA signal, which is present in cytoplasm. When certain RNA molecule with two aptamer domains is present in cells, their cognate aptamer binding proteins can thus aggregate together. | ||
| + | |||
| + | [[Image:12SJTU RNASIGNAL.jpg|thumb|650px|center|''Fig.3'' : | ||
<br> | <br> | ||
| + | '''A''': Sketch of signal RNA molecule (called D0) which consists of PP7 and MS2 aptamer domains. PP7 aptamer binding protein could dimerize with PP7 RNA aptamer domain; MS2 aptamer binding protein could dimerize with MS2 RNA aptamer domain. | ||
| + | <br> | ||
| + | '''B''': Through fusing PP7 and MS2 aptamer binding protein to Membrane Anchor, RNA molecule in Figure A can function as a bridge to connect different proteins, thus decreasing distance between corresponding proteins.]] | ||
| - | === | + | By now, if we want to assemble certain proteins through external signal, we need to find corresponding signal-induced dimer or oligomer. But if we place RNA D0 (with PP7 and MS2 aptamer domains) under various promoters regulated by different signals, approaches to induce dimerization would be expanded sharply. Thus, ''Membrane Rudder'' could sense much more signals. |
| + | |||
| + | We have constructed the PP7 and MS2 standardized BioBrick part, [http://partsregistry.org/Part:BBa_K771111 Part:BBa_K771111] and [http://partsregistry.org/Part:BBa_K771112 Part:BBa_K771112], respectively. | ||
| + | |||
| + | ===Chemical & Peptide Signal=== | ||
| + | There are many ligand-induced dimers in nature. ErbB family proteins are typically ligand-induced dimers. Estrogen receptors only form dimer when estrogen is present. If incorporated into our system, it might have significant application prospect. BMP receptors could form heterodimer in the presence of their cognate peptide ligand. | ||
| + | |||
| + | Many other receptors could also form chemical-signal induced dimers. | ||
| + | |||
| + | Recruiting those chemical and peptide induced dimers could easily broaden the application field of ''Membrane Rudder''. | ||
| + | |||
| + | ==Construction Details== | ||
| - | + | The construction strategy of ''Membrane Rudder'' is similar to that of ''Membrane Accelerator''. The only difference is that signal induced dimers are introduced in Membrane Anchors so that the aggregation state of Membrane Anchors could be dynamically controlled. | |
| - | + | [[File:12SJTU_overview4.gif|center|400px]] | |
| - | + | <center>''Fig.4'': Sketch of ''Membrane Rudder''</center> | |
| - | + | Through controlling the aggregation state of Membrane Anchor linked with enzymes, we can alter the direction of branched biochemical reactions. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | == | + | ==Application== |
| + | What we want to offer is a universal tool which can be linked to different enzymes and signal sensors. | ||
| + | We chose blue light sensor protein -- VVD to test the feasibility of ''Membrane Rudder'' device because of the uniqueness of light signal. | ||
| - | [[ | + | [[Team:SJTU-BioX-Shanghai/Project/project2.1|Violacein and deoxyviolacein synthetic pathway]], a branched enzymatic reaction is recruited to prove that ''Membrane Rudder'' sensing blue light could work as expected. The experiment result confirmed the feasibility of ''Membrane Rudder'' in direction alteration. |
| - | + | ||
| - | + | ||
| - | + | ==Reference== | |
| - | + | ||
| - | + | 1.Delebecque, C. J., A. B. Lindner, et al. (2011). "Organization of intracellular reactions with rationally designed RNA assemblies." Science 333(6041): 470. | |
| + | 2.Kumar, V. and P. Chambon (1988). "The estrogen receptor binds tightly to its responsive element as a ligand-induced homodimer." Cell 55(1): 145. | ||
| - | + | 3.Lemmon, M. A. (2009). "Ligand-induced ErbB receptor dimerization." Experimental cell research 315(4): 638-648. | |
| - | + | 4.Terrillon, S. and M. Bouvier (2004). "Roles of G-protein-coupled receptor dimerization." EMBO reports 5(1): 30-34. | |
| - | + | 5.Wang, X., X. Chen, et al. (2012). "Spatiotemporal control of gene expression by a light-switchable transgene system." Nature Methods. | |
| - | + | ||
| - | |||
<!----------------------------------------------------end---------------------------------> | <!----------------------------------------------------end---------------------------------> | ||
{{Template:12SJTU_footer}} | {{Template:12SJTU_footer}} | ||
Latest revision as of 17:01, 26 October 2012
| ||
|
 "
"