Team:SJTU-BioX-Shanghai/Project/project1.3
From 2012.igem.org
(Difference between revisions)
AleAlejandro (Talk | contribs) (→Light Signal) |
AleAlejandro (Talk | contribs) (→Membrane Rudder) |
||
| Line 31: | Line 31: | ||
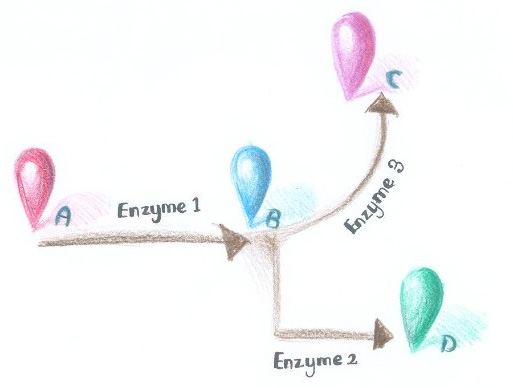
[[Image:12SJTU_divergentreaction.jpg|thumb|500px|center|''Fig.1'' :Demonstration of branched reactions :When signal that induces the dimerization of Enzyme Complex 1 and 2 is present, they would dimerize, making product D more dominant. On the contrary, when signal that induces the dimerization of Enzyme Complex 1 and 3 is present, they would aggregate, so product C is more dominant.]] | [[Image:12SJTU_divergentreaction.jpg|thumb|500px|center|''Fig.1'' :Demonstration of branched reactions :When signal that induces the dimerization of Enzyme Complex 1 and 2 is present, they would dimerize, making product D more dominant. On the contrary, when signal that induces the dimerization of Enzyme Complex 1 and 3 is present, they would aggregate, so product C is more dominant.]] | ||
| - | ''Membrane Accelerator'' employed constitutively dimerizing proteins to assemble enzymes. Imagine if we replace those constitutively dimerizing proteins with signal-induced dimers, which could | + | ''Membrane Accelerator'' employed constitutively dimerizing proteins to assemble enzymes. Imagine if we replace those constitutively dimerizing proteins with signal-induced dimers, which could act as signal sensor, then it is possible to dynamically control the direction of branched reactions. |
| - | There are indeed many signal induced dimers that commonly exist in nature. | + | There are indeed many signal induced dimers that commonly exist in nature, which could sense light, chemical, peptide or even RNA signal. |
<br> | <br> | ||
| Line 44: | Line 44: | ||
Vivid(VVD) protein, photoreceptor of ''Neurospora crassa'' can form dimer in the presence of blue light and disassociate as light is off. Besises, VVD protein belongs to the Per-Arnt-Sim(PAS) protein superfamily. | Vivid(VVD) protein, photoreceptor of ''Neurospora crassa'' can form dimer in the presence of blue light and disassociate as light is off. Besises, VVD protein belongs to the Per-Arnt-Sim(PAS) protein superfamily. | ||
| - | + | VVD mutant (C71V and N56K) is harder to dimerize in the dark and easier to dimerize under blue light compared with wildtype VVD. So this mutant is ideal as light sensor in ''Membrane Rudder'' device. | |
| - | VVD mutant (C71V and N56K) is harder to dimerize in the dark and easier to | + | |
==Transcription Signal== | ==Transcription Signal== | ||
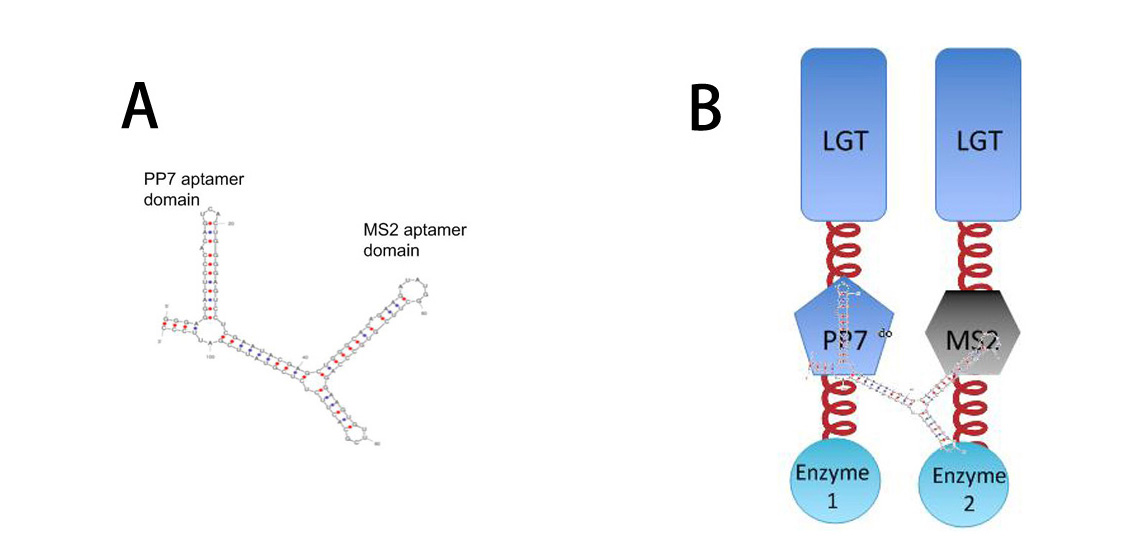
| - | [[Image:12SJTU RNASIGNAL.jpg|thumb| | + | [[Image:12SJTU RNASIGNAL.jpg|thumb|400px|center|''Fig.10'' :A: Sketch of signal RNA molecule which consists of PP7 and MS2 aptamer domains. |
| - | B: Through fusing PP7 and MS2 protein to our membrane device, | + | B: Through fusing PP7 and MS2 protein to our membrane device, RNA molecule in Figure A can function as a bridge to connect different proteins, thus decreasing distance between corresponding proteins.]] |
| - | So far, | + | So far, ''Membrane Accelerator'' and ''Membrane Rudder'' is generally about posttranslational control over the host cell. To connect our relatively isolated system to transcription level, we employed RNA signal, which is present in cytoplasm. When certain RNA molecule with dimerization domain is present in cells, its cognate binding proteins can thus dimerize with each other, accelerating certain pathway. |
| + | |||
| - | |||
| - | |||
We incorporated RNA molecule with PP7 and MS2 aptamer domains that bind PP7 and MS2 aptamer binding proteins. (Delebecque, Lindner et al. 2011) | We incorporated RNA molecule with PP7 and MS2 aptamer domains that bind PP7 and MS2 aptamer binding proteins. (Delebecque, Lindner et al. 2011) | ||
Revision as of 21:14, 26 September 2012
 "
"