Team:Penn/Notebook/Biofilms
From 2012.igem.org
(Difference between revisions)
(→7/2/12) |
(→7/3/12) |
||
| Line 70: | Line 70: | ||
==7/3/12== | ==7/3/12== | ||
| - | * | + | *Ran gel of ligated product, no size shift, unlikely that ligation was successful. |
| + | *Ligation rxn transformed to make sure | ||
==7/5/12== | ==7/5/12== | ||
Revision as of 19:18, 17 July 2012
Contents |
6/6/12
- Set up equipment
- Autoclaved 2x 1L Sterile ddH2O
- Autoclaved 500 mL LB Broth for scale up of luxS (Plate 3 Well 2H, pSB1A2), plsr (Plate 2 Well 14H in pSB1A2 resistance to Amp)
- Added iGEM logo to wiki
6/7/12
- Resuspended available biobricks luxS & plsr (resuspended in 10uL ddH2O)
- Resuspended DNA transformed into DH5α (40uL DH5α+2uL DNA) & plated on LB plates w/ 15 uL of 100 mg/mL Amp spread on surface
- Requested lsrR & lsrK from iGEM HQ
6/8/12
- iGEM Wiki now split into two separate Notebooks
- Plenty of colonies, too many to count(TMTC) grew from plsr & luxS transformation from 6/7/12
- Selected one colony each
- Grew in 10 mL LB+Amp (100 ug/mL)
- Contacted researchers for V. harveyi bioassay & lysostaphin, V. harveyi must be purchased through ATCC ($300), lysostaphin obtained thru MTA.
6/9/12
- ~2mL of LB evaporated during incubation
- Cells spun down @ 5000 rpm for 10 min
- Miniprepped 4x Lysis into 1 column
- [plsr]=112 ng/uL
- [luxS]=114 ng/uL
6/11/12-6/16/12
- Dry lab work
6/18/12
- Obtained & resuspended primers for eGFP & plsr
6/19/12
- performed PCR w/ NEB standard taq kit w/ primers for eGFP & plsr
- Tested 2 annealing temperatures, 55C and 50C
- Ran gel w/ PCR product (2% agarose), saw clear eGFP bands, but possible .1kb obscured by loading dye.
6/20/12
- Performed qiagen PCR purification & nanodropped (it like it's hot)
6/21/12
- Ran gel of yesterday's PCR purification
- EtBr Gel 2% Agarose
- Lane 1: NEB 2-log ladder (1 ug loaded)
- Lane 2: PCR of eGFP using plsr_XhoI Fwd & eGFP_EcoRI Rev primers w/ 55C annealing temp.
- Lane 3: PCR of plsr using plsr_BglII Fwd & plsr_XhoI Rev primers w/ 55C annealing temp.
- Lane 4: PCR of eGFP using plsr_XhoI Fwd & eGFP_EcoRI Rev primers w/ 50C annealing temp.
- Lane 5: PCR of plsr using plsr_BglII Fwd & plsr_XhoI Rev primers w/ 50C annealing temp.
- Lane6/7: Null
- Lane 8: 1kb ladder left at rt o/n
- EtBr Gel 2% Agarose
- Obtained & transformed pET-26b
6/22/12
- Miniprepped pet26b and digested with BglII and EcorR
6/25/12
- Ran gel of eGFP/plsr ligation
- Ordered restriction enzymes from the Cell Center
6/26/12
- Attempted Gel Purification of digest products from 2/25/12
- Yields for Gel purification were too low to be useful (>1 ng/uL)
- Digested purified PCR rxns (annealing temperature=55C)
6/27/12
Ran gel of digested PCR rxns (55C), found no evidence of successful digestion.
6/28/12
Purified digest and attempted
6/29/12
- Performed digest of pET-26b, eGFP, and plsr
- Spread Biobrick shipment on Amp plates (lsrR, lsrK)
7/2/12
- Selected colonies from lsrK and lsrR streaked plates
- Grew in 10mL TB Broth
7/3/12
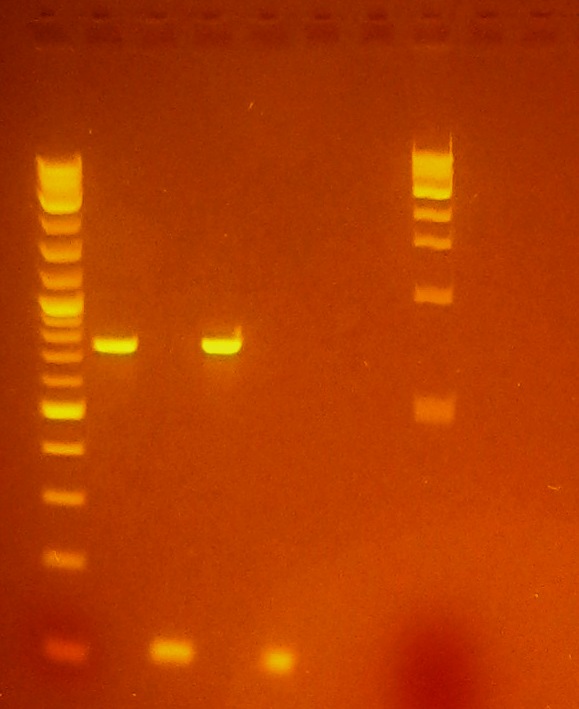
- Ran gel of ligated product, no size shift, unlikely that ligation was successful.
- Ligation rxn transformed to make sure
7/5/12
Unknown
7/6/12
- Redesigned primers for plsr & egfp
 "
"