Team:Peking/Project/Overview
From 2012.igem.org
Spring zhq (Talk | contribs) |
|||
| (75 intermediate revisions not shown) | |||
| Line 10: | Line 10: | ||
<h3 id="title1">Achievements</h3> | <h3 id="title1">Achievements</h3> | ||
<p> | <p> | ||
| - | + | Here is a list of our achievements from this summer.<br /><BR> | |
| - | 1. | + | 1. We helped four other iGEM teams by sharing DNA materials, characterizing their parts and modeling! Click <a href="/Team:Peking/HumanPractice/Outreach/Collaboration"><i> Here</i></a><br /><br /> |
| - | 2. | + | 2. We outlined in detail a new approach of human pratice called "Sowing Tomorrow's Synthetic Biologists"! Click <a href="/Team:Peking/HumanPractice/Sowing"><i> Here</i></a><br /><br /> |
| + | 3. We presented all fresh iGEMers with a collection and praise of historic iGEM projects to share and learn from each other!Click <a href="/Team:Peking/HumanPractice/Review"><i> Here</i></a><br /><br /> | ||
| + | 4. We submitted 11 high quality and well-characterized standard biobricks! Click <a href="/Team:Peking/DataPage"><i> Here</i></a><br /><br /> | ||
| + | 5. We improved the ease-of-use of "Lux Brick" that primarily constructed by Cambridge iGEM 2010 and carefully characterized its dynamics of functionality! Click <a href="/wiki/index.php?title=Team:Peking/Project/Communication/Results#title2"><i> Here</i></a> or <a href="http://partsregistry.org/Part:BBa_K819008">MainPage</a><br /><br /> | ||
| + | 6. We successfully implemented spatiotemporal control of cellular behavior, such as high-resolution 2-D and 3-D bio-printing using dim light, and even utilizing the luminescence of an iPad! Click <a href="/Team:Peking/Project/3D/2D"><i> Here</i></a><br /><br /> | ||
| + | 7. We successfully implemented cell-cell communication using bioluminescence for the very first time in synthetic biology! Click <a href="/Team:Peking/Project/Communication/Results"><i> Here</i></a><br /><br /> | ||
| + | We deserve a <b>Gold Medal Prize</b>.<br /> | ||
</p> | </p> | ||
</div> | </div> | ||
<div class="PKU_context floatR"> | <div class="PKU_context floatR"> | ||
| - | <h3 id="title2">1. Luminesensor < | + | <h3 id="title2">1. The <i>Luminesensor</i> <br> -- An Ultrasensitive Light Sensor</h3> |
<p> | <p> | ||
| - | Based on | + | Based on comprehensive literature reviews, our team has concluded that an ultra-sensitive light-sensing light-off system which constructed by 3 our ECUST members from Yang lab, here we called the <i>Luminesensor</i> may circumvent serious issues of current optogenetic methods, e.g. cytotoxicity, narrow dynamic range, and dependency on laser and exogenous chromophores. |
<br /> <br /> | <br /> <br /> | ||
| - | + | </p> | |
| + | <p> | ||
| + | Amazingly, the <i>Luminesensor</i> proved to be sensitive to natural light and even bioluminescence. Moreover, the <i>Luminesensor</i> is able to respond to light signals with broad illuminance scales (Figure 1). | ||
</p> | </p> | ||
<div class="floatC"> | <div class="floatC"> | ||
| - | <img src="/wiki/images/ | + | <img src="/wiki/images/d/d0/Peking2012_IlluminanceScales.png" alt="" style="width:600px;" /> |
<div> | <div> | ||
<p class="description"> | <p class="description"> | ||
| - | Figure 1. The | + | Figure 1. The broad illuminance scales to which <i>Luminesensor</i> could respond to. |
</p> | </p> | ||
</div> | </div> | ||
</div> | </div> | ||
| + | </div> | ||
| + | <div class="PKU_context floatR"> | ||
| + | <h3 id="title3">2. Synthetic Biology in 2D and 3D <br /> -- A Practical Application</h3> | ||
<p> | <p> | ||
| - | + | Few optogenetics methods have been applied to practical applications until now. To fully demonstrate spatiotemporally precise manipulation of cellular behavior using our <i>Luminesensor</i>, our team has deeply explored the applications in 2D and 3D bio-printing. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
<br /> <br /> | <br /> <br /> | ||
| - | + | High resolution images could be easily obtained by 2D bioprinting on a bacterial lawn bearing the <i>Luminesensor</i> (Figure 2). (more on <a href="/Team:Peking/Project/3D/2D">2D Printing</a>). | |
</p> | </p> | ||
<div class="floatC"> | <div class="floatC"> | ||
| - | <img src="/wiki/images/ | + | <img src="/wiki/images/c/ca/Peking2012_PrintParagraph.jpg" alt="" style="width:500px;" /> |
<div> | <div> | ||
<p class="description"> | <p class="description"> | ||
| - | Figure 2. | + | Figure 2. High resolution of 2D bioprinting. |
</p> | </p> | ||
</div> | </div> | ||
</div> | </div> | ||
<p> | <p> | ||
| - | + | What's more, sharp images could be printed using an Apple iPad as light source (see <a href="/Team:Peking/Project/3D/2D">iPrinting</a>), which indicates that our <i>Luminesensor</i> could serve as the interface between biological systems and electrical devices. | |
| + | <br /> <br /> | ||
| + | A very promising application of the <i>Luminesensor</i> is 3D printing, using light signals of spatially high resolution. Three dimensional images could be elegantly printed into media mixed with bacteria expressing the <i>Luminesensor</i> (see <a href="/Team:Peking/Project/3D/3D">3D Printing</a>). A new design strategy of 3D printer using holographic technology instead of layer-by-layer printing, was also proposed. With such a power tool, 3D printing regarding biological material has endless potentials for medical use (crazy ideas in <a href="/Team:Peking/Project/3D/Future">Future</a>). | ||
</p> | </p> | ||
</div> | </div> | ||
<div class="PKU_context floatR"> | <div class="PKU_context floatR"> | ||
| - | <h3 id=" | + | <h3 id="title4">3. Cell-Cell Light Communication <br /> -- A Novel Strategy of Signaling</h3> |
<p> | <p> | ||
| - | + | Cell-cell communication based on quorum sensing systems, e.g. AHL, has been largely utilized by synthetic biologists to construct gene circuits with complex functions, e.g. pattern formation and edge detection. However, the diffusion and saturation of quorum sensing chemicals limit the delivery of communication signals, which in turn requires high spatiotemporal resolution and long range interaction. | |
<br /> <br /> | <br /> <br /> | ||
| - | + | The ultrasensitivity of the <i>Luminesensor</i> encouraged our team to explore the possibility of cell-cell light communication (Figure 3). By employing bacteria expressing the lux operon from <i>V. fisheri</i> as "light senders" and those bearing the <i>Luminesensor</i> as "light receiver", we successfully demonstrated, for the first time ever, that light communication between cells could be achieved beyond direct physical contact (see <a href="/Team:Peking/Project/Communication/Results">video</a> in Results). Through measuring the dynamics of light communication, it was proved that bioluminescence was sufficient to trigger the response of the <i>Luminesensor</i> (data in <a href="/Team:Peking/Project/Communication/Results">Results</a>). | |
</p> | </p> | ||
<div class="floatC"> | <div class="floatC"> | ||
| - | <img src="/wiki/images/ | + | <img src="/wiki/images/9/9c/Peking2012_overview_achieve_communication.png" alt="" style="width:500px;" /> |
<div> | <div> | ||
<p class="description"> | <p class="description"> | ||
| - | Figure 3. | + | Figure 3. The concept of cell-cell communication through light. |
</p> | </p> | ||
</div> | </div> | ||
</div> | </div> | ||
<p> | <p> | ||
| - | + | Our Light-Off system could be upgraded into Light-On system through simple design (details in <a href="/Team:Peking/Project/Communication/Design#title3">Design</a>), which enables us to achieve more flexible cell-cell light communication (creative ideas in <a href="/Team:Peking/Project/Communication/Future">Future</a>). | |
| - | + | ||
| - | + | ||
</p> | </p> | ||
</div> | </div> | ||
<div class="PKU_context floatR"> | <div class="PKU_context floatR"> | ||
| - | <h3 id=" | + | <h3 id="title5">4. Phototaxis <br /> --Controlling Cell Motion by Light</h3> |
<p> | <p> | ||
| - | + | What makes <i>Luminesensor</i> outstanding is not only its ultra-sensitivity, but also the particularly high dynamic range (data on <a href="/Team:Peking/Project/Luminesensor/Characterization#title3">Characterization</a>). Such switch-like property makes <i>Luminesensor</i> a very reliable and beneficial module in synthetic biology. Similar to the light-controlled animal behavior in neuroscience, by coupling <i>Luminesensor</i> with endogenous systems, it is possible to integrate the light signal with cell motion. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
</p> | </p> | ||
| - | < | + | <div class="floatC"> |
| - | < | + | <img src="/wiki/images/c/c1/Peking2012_Home_Phototaxis.jpg" alt="" style="width:500px;" /> |
| - | + | <div> | |
| + | <p class="description"> | ||
| + | Figure 4. The concept of phototaxis behavior. | ||
| + | </p> | ||
| + | </div> | ||
| + | </div> | ||
<p> | <p> | ||
| - | + | "Phototactic" bacteria can be built by reprogramming the chemotaxis system in <i>E. coli</i> through light with the <i>Luminesensor</i> (Figure 4). By controlling the expression of the CheZ protein with light, the tumbling frequency is coupled to intensity of light signals (details in <a href="/Team:Peking/Project/Phototaxis/Design">Design</a>). On the border of the light and dark fields, motility ability of the cells in a single colony on the two sides is sufficient to result in an uneven colony (see <a href="/Team:Peking/Project/Phototaxis/Demonstration">Demonstration</a>). Light-controlled cellular motion indeed has very promising environmental and medical applications for the future, e.g. the delivery of drugs to target concerns. | |
| - | + | ||
| - | + | ||
</p> | </p> | ||
</div> | </div> | ||
</html>{{Template:Peking2012_Color_Epilogue}} | </html>{{Template:Peking2012_Color_Epilogue}} | ||
Latest revision as of 07:48, 16 October 2012
Achievements
Here is a list of our achievements from this summer.
1. We helped four other iGEM teams by sharing DNA materials, characterizing their parts and modeling! Click Here
2. We outlined in detail a new approach of human pratice called "Sowing Tomorrow's Synthetic Biologists"! Click Here
3. We presented all fresh iGEMers with a collection and praise of historic iGEM projects to share and learn from each other!Click Here
4. We submitted 11 high quality and well-characterized standard biobricks! Click Here
5. We improved the ease-of-use of "Lux Brick" that primarily constructed by Cambridge iGEM 2010 and carefully characterized its dynamics of functionality! Click Here or MainPage
6. We successfully implemented spatiotemporal control of cellular behavior, such as high-resolution 2-D and 3-D bio-printing using dim light, and even utilizing the luminescence of an iPad! Click Here
7. We successfully implemented cell-cell communication using bioluminescence for the very first time in synthetic biology! Click Here
We deserve a Gold Medal Prize.
1. The Luminesensor
-- An Ultrasensitive Light Sensor
Based on comprehensive literature reviews, our team has concluded that an ultra-sensitive light-sensing light-off system which constructed by 3 our ECUST members from Yang lab, here we called the Luminesensor may circumvent serious issues of current optogenetic methods, e.g. cytotoxicity, narrow dynamic range, and dependency on laser and exogenous chromophores.
Amazingly, the Luminesensor proved to be sensitive to natural light and even bioluminescence. Moreover, the Luminesensor is able to respond to light signals with broad illuminance scales (Figure 1).
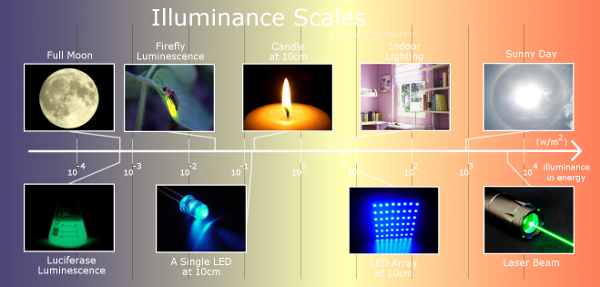
Figure 1. The broad illuminance scales to which Luminesensor could respond to.
2. Synthetic Biology in 2D and 3D
-- A Practical Application
Few optogenetics methods have been applied to practical applications until now. To fully demonstrate spatiotemporally precise manipulation of cellular behavior using our Luminesensor, our team has deeply explored the applications in 2D and 3D bio-printing.
High resolution images could be easily obtained by 2D bioprinting on a bacterial lawn bearing the Luminesensor (Figure 2). (more on 2D Printing).

Figure 2. High resolution of 2D bioprinting.
What's more, sharp images could be printed using an Apple iPad as light source (see iPrinting), which indicates that our Luminesensor could serve as the interface between biological systems and electrical devices.
A very promising application of the Luminesensor is 3D printing, using light signals of spatially high resolution. Three dimensional images could be elegantly printed into media mixed with bacteria expressing the Luminesensor (see 3D Printing). A new design strategy of 3D printer using holographic technology instead of layer-by-layer printing, was also proposed. With such a power tool, 3D printing regarding biological material has endless potentials for medical use (crazy ideas in Future).
3. Cell-Cell Light Communication
-- A Novel Strategy of Signaling
Cell-cell communication based on quorum sensing systems, e.g. AHL, has been largely utilized by synthetic biologists to construct gene circuits with complex functions, e.g. pattern formation and edge detection. However, the diffusion and saturation of quorum sensing chemicals limit the delivery of communication signals, which in turn requires high spatiotemporal resolution and long range interaction.
The ultrasensitivity of the Luminesensor encouraged our team to explore the possibility of cell-cell light communication (Figure 3). By employing bacteria expressing the lux operon from V. fisheri as "light senders" and those bearing the Luminesensor as "light receiver", we successfully demonstrated, for the first time ever, that light communication between cells could be achieved beyond direct physical contact (see video in Results). Through measuring the dynamics of light communication, it was proved that bioluminescence was sufficient to trigger the response of the Luminesensor (data in Results).
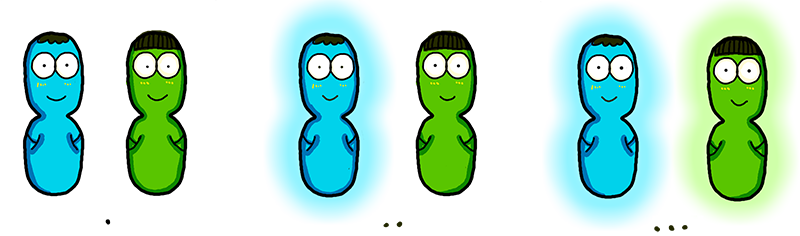
Figure 3. The concept of cell-cell communication through light.
Our Light-Off system could be upgraded into Light-On system through simple design (details in Design), which enables us to achieve more flexible cell-cell light communication (creative ideas in Future).
4. Phototaxis
--Controlling Cell Motion by Light
What makes Luminesensor outstanding is not only its ultra-sensitivity, but also the particularly high dynamic range (data on Characterization). Such switch-like property makes Luminesensor a very reliable and beneficial module in synthetic biology. Similar to the light-controlled animal behavior in neuroscience, by coupling Luminesensor with endogenous systems, it is possible to integrate the light signal with cell motion.
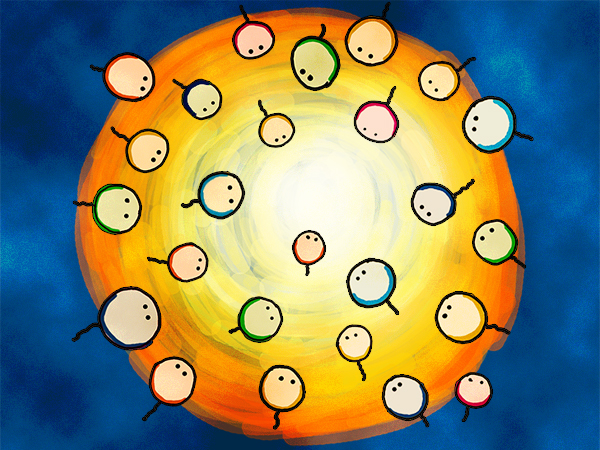
Figure 4. The concept of phototaxis behavior.
"Phototactic" bacteria can be built by reprogramming the chemotaxis system in E. coli through light with the Luminesensor (Figure 4). By controlling the expression of the CheZ protein with light, the tumbling frequency is coupled to intensity of light signals (details in Design). On the border of the light and dark fields, motility ability of the cells in a single colony on the two sides is sufficient to result in an uneven colony (see Demonstration). Light-controlled cellular motion indeed has very promising environmental and medical applications for the future, e.g. the delivery of drugs to target concerns.
 "
"














