Team:Peking/Project/Communication
From 2012.igem.org
m |
|||
| (12 intermediate revisions not shown) | |||
| Line 12: | Line 12: | ||
<h3 class="title1">Introduction</h3> | <h3 class="title1">Introduction</h3> | ||
<p> | <p> | ||
| - | As a significant component in signal transduction, cell-cell communication has fueled numerous biological researches | + | |
| - | However, it is difficult for these systems to perform long-distance signaling, such as synchronizing cells in a large population due to the short-range diffusion of chemicals. But an even more serious issue is the basis of synthetic systems on quorum sensing signals, which are | + | |
| - | As demonstrated above | + | As a significant component in signal transduction, cell-cell communication has fueled numerous biological researches. Among them is the discovery of quorum sensing signals, <i>e.g.</i> AHL and AIP. During the last decade, many insightful and valuable synthetic biology projects have been constructed to perform complex functions based on cell-cell communication, <i>e.g.</i> pattern formation and synthetic ecosystems. <br /><br /> |
| + | However, it is difficult for these systems to perform long-distance signaling, such as in the case of synchronizing cells in a large population, due to the short-range diffusion of chemicals. ' | ||
| + | |||
| + | But an even more serious issue is the basis of synthetic systems on quorum sensing signals, which are difficult to reset because the chemicals are easily saturated in many cases. Additionally, it is difficult to achieve inter-kingdom communication through quorum sensing signals due to the fact that the transcription machinery of prokaryotes and eukaryotes are dramatically different. <br /><br /> | ||
| + | |||
| + | As demonstrated above, the ultrasensitive <i>Luminesensor</i> is able to respond to very dim light and maintains a wide dynamic range. All of this encouraged our team to explore the possibility of cell-cell communication through light. The delivery of light signals is not limited by diffusion or by a variety in organisms across species or even kingdoms. By carefully selecting the lux operon (bacterial luciferase) as the light sender module, we have successfully demonstrated that the <i>Luminesensor</i> is able to sense the blue light produced by bacterial luciferase. This is the very first time that light-communication between cells has been achieved without direct physical contact. As a proof of concept, a video was recorded to reveal the course of change of both the sender and the receiver cells. Quantitative data was also obtained to evaluate the efficiency of light-communication. To build a complete light-communication system, a Light-On system was also proposed to achieve both positive and negative control by light. As the application of synthetic biology is coming of age, we have probed into the bright future of light-communication. | ||
</p> | </p> | ||
| + | <div class="floatC"> | ||
| + | <img src="/wiki/images/f/f7/Peking2012_light_communication_quickproof2.png" alt="Figure 1" style="width:500px"/> | ||
| + | <p class="description">Figure 1. The treatment to each group(upper) and the result(lower). 1 and 2 contain cells grown in the communication set-up, but for group 2 there is no light emitting cell in the conical flask but only wild-type DH5α. 3 and 4 contain the same light sensing cells grown in test tubes under dim LED (3) or in pure darkness (4).</p> | ||
| + | </div> | ||
| + | <p> | ||
| + | As a hallmark of coordinated cellular behavior, pattern formation typically required cell-cell communication and intracellular signal processing. For more site-specific signaling and pattern formation, light may be more appropriate alternative. Due to the high sensitivity of our <i>Luminesensor</i>, it is possible to construct a ring-like pattern based on light-communication, previously done by AHL. This kind of flexibility enables its application in tissue-engineering, bio-sensing and bio-manufacturing. (See <a href="/Team:Peking/Modeling/Ring">Modeling Ring Pattern</a>)<br /> | ||
| + | </p> | ||
| + | <div class="floatC"> | ||
| + | <img src="/wiki/images/c/cf/Band_Origin_3.png" alt="" style="width:500px;"/> | ||
| + | <p class="description"> | ||
| + | Figure 2. Ring Pattern Formation. | ||
| + | </p> | ||
| + | </div> | ||
| + | <p> | ||
| + | The high sensitivity of <i>Luminesensor</i> extends its field applications and enables us to achieve light-communication between E. coli cells. As demonstrations, we constructed and ring-like pattern in E. coli based on light-communication between cells. There are two kinds of E. coli cells on the plate: sender cells and receiver cells. Sender cells in the center of the plate produce blue light (490nm) on the addition of arabinose. Then receiver cells around the senders receive the light and react differently to the different light intensity. A high threshold and a low threshold are created in the system, between which the GFP are activated <sup><a href="#ref1" title="Subhayu Basu et al.(2005), A synthetic multicellular system for programmed pattern formation. Nature, vol.434: 1130: 1134">[1]</a></sup>. | ||
| + | </p> | ||
| + | </div> | ||
| + | |||
| + | <div class="PKU_context floatR"> | ||
| + | <h3 id="title2">Reference</h3> | ||
| + | <p></p> | ||
| + | <ul class="refer"> | ||
| + | <li id="ref1"> | ||
| + | 1. Subhayu Basu et al.(2005), A synthetic multicellular system for programmed pattern formation. <i>Nature</i>, vol.434: 1130: 1134 | ||
| + | </li></ul> | ||
</div> | </div> | ||
</html> | </html> | ||
Latest revision as of 03:27, 26 October 2012
Introduction
As a significant component in signal transduction, cell-cell communication has fueled numerous biological researches. Among them is the discovery of quorum sensing signals, e.g. AHL and AIP. During the last decade, many insightful and valuable synthetic biology projects have been constructed to perform complex functions based on cell-cell communication, e.g. pattern formation and synthetic ecosystems.
However, it is difficult for these systems to perform long-distance signaling, such as in the case of synchronizing cells in a large population, due to the short-range diffusion of chemicals. '
But an even more serious issue is the basis of synthetic systems on quorum sensing signals, which are difficult to reset because the chemicals are easily saturated in many cases. Additionally, it is difficult to achieve inter-kingdom communication through quorum sensing signals due to the fact that the transcription machinery of prokaryotes and eukaryotes are dramatically different.
As demonstrated above, the ultrasensitive Luminesensor is able to respond to very dim light and maintains a wide dynamic range. All of this encouraged our team to explore the possibility of cell-cell communication through light. The delivery of light signals is not limited by diffusion or by a variety in organisms across species or even kingdoms. By carefully selecting the lux operon (bacterial luciferase) as the light sender module, we have successfully demonstrated that the Luminesensor is able to sense the blue light produced by bacterial luciferase. This is the very first time that light-communication between cells has been achieved without direct physical contact. As a proof of concept, a video was recorded to reveal the course of change of both the sender and the receiver cells. Quantitative data was also obtained to evaluate the efficiency of light-communication. To build a complete light-communication system, a Light-On system was also proposed to achieve both positive and negative control by light. As the application of synthetic biology is coming of age, we have probed into the bright future of light-communication.
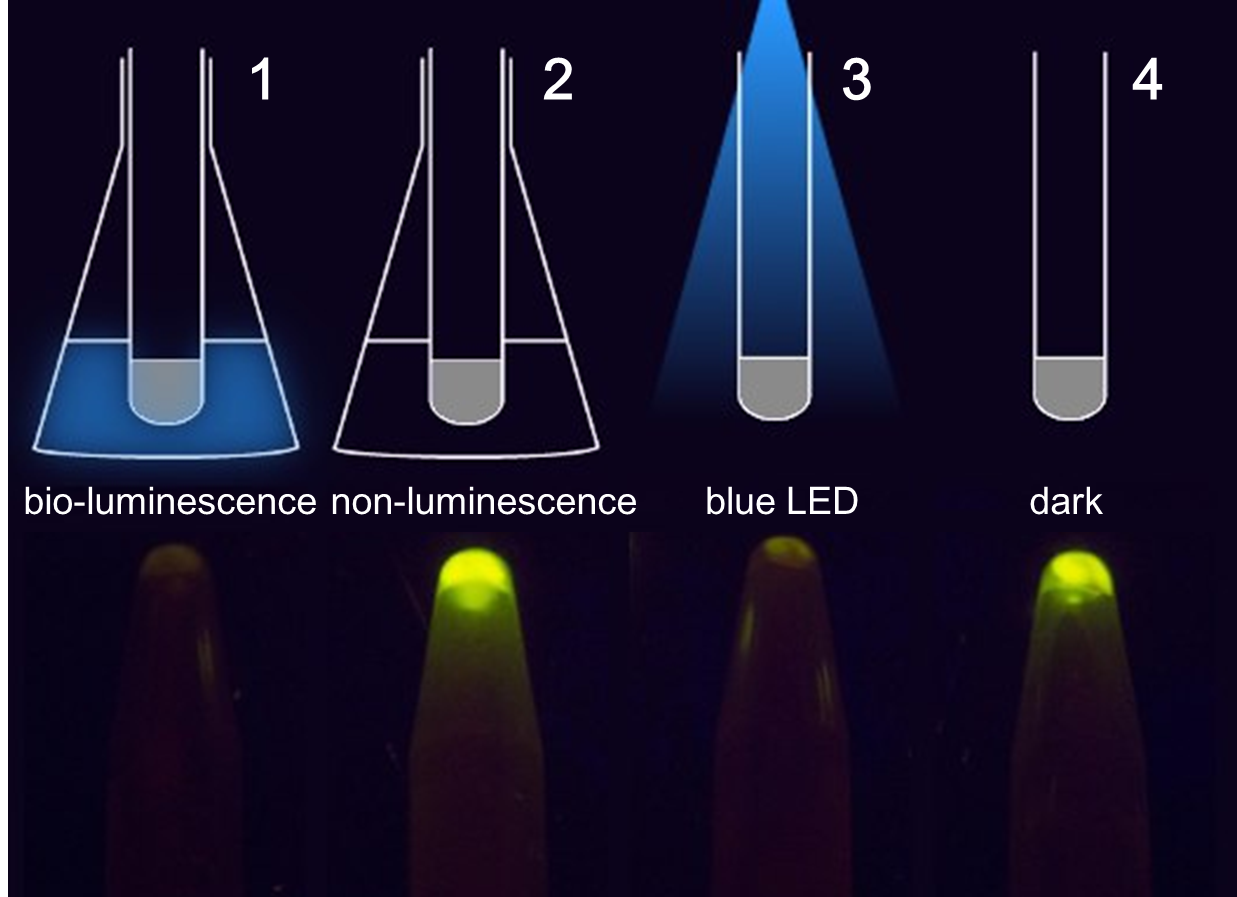
Figure 1. The treatment to each group(upper) and the result(lower). 1 and 2 contain cells grown in the communication set-up, but for group 2 there is no light emitting cell in the conical flask but only wild-type DH5α. 3 and 4 contain the same light sensing cells grown in test tubes under dim LED (3) or in pure darkness (4).
As a hallmark of coordinated cellular behavior, pattern formation typically required cell-cell communication and intracellular signal processing. For more site-specific signaling and pattern formation, light may be more appropriate alternative. Due to the high sensitivity of our Luminesensor, it is possible to construct a ring-like pattern based on light-communication, previously done by AHL. This kind of flexibility enables its application in tissue-engineering, bio-sensing and bio-manufacturing. (See Modeling Ring Pattern)
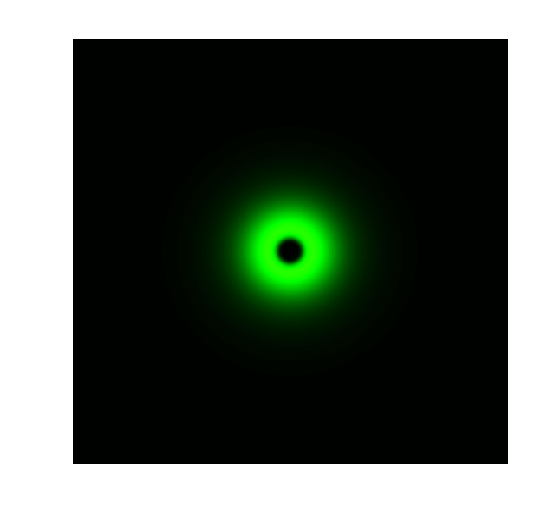
Figure 2. Ring Pattern Formation.
The high sensitivity of Luminesensor extends its field applications and enables us to achieve light-communication between E. coli cells. As demonstrations, we constructed and ring-like pattern in E. coli based on light-communication between cells. There are two kinds of E. coli cells on the plate: sender cells and receiver cells. Sender cells in the center of the plate produce blue light (490nm) on the addition of arabinose. Then receiver cells around the senders receive the light and react differently to the different light intensity. A high threshold and a low threshold are created in the system, between which the GFP are activated [1].
Reference
- 1. Subhayu Basu et al.(2005), A synthetic multicellular system for programmed pattern formation. Nature, vol.434: 1130: 1134
 "
"










