Team:Peking/DataPage
From 2012.igem.org
m |
|||
| (41 intermediate revisions not shown) | |||
| Line 14: | Line 14: | ||
</style> | </style> | ||
<div class="PKU_context floatR first"> | <div class="PKU_context floatR first"> | ||
| - | <h3 id= | + | <h3 id="title2">Data for Favorite Parts</h3> |
<p> | <p> | ||
| - | <b> | + | An <b>Constitutive LuxBrick Generator(<a href="http://partsregistry.org/Part:BBa_K819005">Main Page-BBa_K819005</a>)</b> |
| + | <br /><br /><b>Testing device for the <b><i>Luminesensor</i></b> (<a href="http://partsregistry.org/Part:BBa_K819006">Main Page-BBa_K819006</a>) and measurement device for the Luminesensor (<a href="http://partsregistry.org/Part:BBa_K819007">Main Page-BBa_K819007</a>)</b> a fast degrading GFP ligated to the downstream of SulA408 promoter and RecA408 promoter, respectively. These two promoters are regulated by the <i>Luminesensor</i> and works independently of the host genetic context. | ||
</p> | </p> | ||
</div> | </div> | ||
<div class="PKU_context floatR"> | <div class="PKU_context floatR"> | ||
| - | <h3 id= | + | <h3 id="title3">Data for Optimized Parts</h3> |
<div class="floatR"> | <div class="floatR"> | ||
<img src="/wiki/images/1/1c/Peking2012_part_T7_operon.png" alt="Fig 1. BL21 cells harboring T7-operon(BBa_K819008) are emitting blue luminescence under IPTG induction" style="width:175px;" /> | <img src="/wiki/images/1/1c/Peking2012_part_T7_operon.png" alt="Fig 1. BL21 cells harboring T7-operon(BBa_K819008) are emitting blue luminescence under IPTG induction" style="width:175px;" /> | ||
| - | <p class="description" style="width:265px;"> | + | <p class="description" style="width:265px;">Figure 2. BL21 cells harboring T7-lux operon(BBa_K819008) are emitting blue luminescence under IPTG induction at 5x10<sup>-5</sup>M</p> |
</div> | </div> | ||
| - | <p><b>Lux Operon under T7 promoter (<a href="http://partsregistry.org/Part:BBa_K819008">BBa_K819008</a>)</b><br/>We successfully improved the easy-of-use of “lux brick” (<a href="http://partsregistry.org/Part:BBa_K325909">BBa_K325909</a>) part constructed by Cambridge 2010 iGEM team by | + | <p><b>Lux Operon under T7 promoter (<a href="http://partsregistry.org/Part:BBa_K819008">Main Page-BBa_K819008</a>)</b><br/>We successfully improved the easy-of-use of “lux brick” (<a href="http://partsregistry.org/Part:BBa_K325909">BBa_K325909</a>) part constructed by Cambridge 2010 iGEM team by placing the coding sequence of luxbrick (luxCDABEG) and related RBS under T7 promoter (<a href="http://partsregistry.org/Part:BBa_I712074">BBa_I712074</a>). |
| - | <br/><br/>Luxbrick under T7 promoter is | + | <br/><br/> T7 promoter separates sensing/circuitry functions from pathways/actuation. It is encoded in genetically distinct regions while linked with other circuits by providing the output for the circuits that drive the expression of phage polymerases. Luxbrick under T7 promoter is very modular, because it is transcribed by T7 polymerase, which can be placed under any other promoter, forming a interface between luxbrick and other systems. |
| - | + | Once transformed into BL21(de3)strain, high protein production can be achieved rapidly after IPTG induction.Final concentration of IPTG should be round 0.05mM. <br /> | |
| - | + | <br/><br/> To note that the incubating temperature should be no higher than 30<sup>o</sup>C, or the heavy Lux complex can easily aggregate. | |
| + | Optimum incubating conditions provided by Peking iGEM 2012: | ||
| + | 250 rpm, 22<sup>o</sup>C, good ventilation after induction(final concentration of IPTG: round 0.5 mM). | ||
</p> | </p> | ||
</div> | </div> | ||
<div class="PKU_context floatR"> | <div class="PKU_context floatR"> | ||
| - | <h3 id= | + | <h3 id="title4">Data for Other Submitted Parts</h3> |
<p> | <p> | ||
| - | + | <b><i>Luminesensor</i> repressible SulA408 promoter (<a href="http://partsregistry.org/Part:BBa_K819017">Main Page-BBa_K819017</a>) <br/><i>Luminesensor</i> repressible RecA408 promoter (<a href="http://partsregistry.org/Part:BBa_K819002">Main Page-BBa_K819002</a>)</b><br />These are the two promoters used in this year’s project to test the functionality of <b><i>Luminesensor</i></b> and measure the efficiency of it. | |
| + | <br /><br /><b>Weak CheZ generator (<a href="http://partsregistry.org/Part:BBa_K819009">Main Page-BBa_K819009</a>)<br/> | ||
| + | medium CheZ generator (<a href="http://partsregistry.org/Part:BBa_K819010">Main Page-BBa_K819010</a>)</b><br />Chemotaxis protein CheZ ligated to 2 constitutive promoters with varying strengths. These two parts express the CheZ protein in varied quantities, which corresponds to the different levels of induced mobility in the ΔCheZ <i>E.coli</i> strain. | ||
| + | <br /><br /><b><i>Luminesensor</i> repressible RecA408 promoter (<a href="http://partsregistry.org/Part:BBa_K819015">Main Page-BBa_K819015</a>)<br/><i>Luminesensor</i> repressible SulA408 promoter (<a href="http://partsregistry.org/Part:BBa_K819016">Main Page-BBa_K819016</a>)</b><br />Chemotaxis protein CheZ ligated to the <b><i>Luminesensor</i></b> repressible SulA408 promoter and RecA408 promoter, respectively. Presumably these two parts can would convert chemotaxis pathway of <i>E.coli</i> into a phototaxis pathway by controlling the expression level of CheZ in ΔCheZ strain in response to blue light. | ||
| + | <br /><br /><b><i>Luminesensor</i> repressible LacIM generator</i> (<a href="http://partsregistry.org/Part:BBa_K819012">Main Page-BBa_K819012</a>)</b><br />Coding sequence of LacI mutant protein driven by the <b><i>Luminesensor</i></b> repressible SulA408 promoter (<a href="http://partsregistry.org/Part:BBa_K819017">BBa_K819017</a>). | ||
</p> | </p> | ||
</div> | </div> | ||
| - | |||
<div class="PKU_context floatR"> | <div class="PKU_context floatR"> | ||
| - | <h3 id= | + | <h3 id="title5">Data for Pre-Existing Parts</h3> |
| - | <p><b>luxbrick (<a href="http://partsregistry.org/Part:BBa_K325909">BBa_K325909</a>)</b> <br/>luxbrick is a bacteria luciferase part constructed by Cambridge 2010 iGEM team. They successfully expressed | + | <p><b>luxbrick (<a href="http://partsregistry.org/Part:BBa_K325909">Main Page-BBa_K325909</a>)</b> <br/>luxbrick is a bacteria luciferase part constructed by Cambridge 2010 iGEM team. They successfully expressed bacterial luciferase in E.coli, which could produce blue light under L-arabinose induction, and they carefully characterized this part. But because there are data we need while they didn’t give, we made the following supplementary characterization.<br /><br />The supplementary characterization and our application of luxbrick part were added to the experience page of this part (see <a href="http://partsregistry.org/Part:BBa_K325909:Experience">Experience-BBa_K325909</a>)<br /><br />The spectrum of the light emitted from <i>E.coli</i> harboring luxbrick was measured and it showed a maximum intensity at 485nm.</p> |
<div class="floatC"> | <div class="floatC"> | ||
| - | <img src="/wiki/images/c/c3/Peking2012_light_communication_3.png" alt="Figure | + | <img src="/wiki/images/c/c3/Peking2012_light_communication_3.png" alt="Figure 3" /> |
| - | <p class="description">Figure | + | <p class="description">Figure 3. TOP10 cells harboring the luxbrick were cultured in LB medium and induced with L-arabinose at 10<sup>-3</sup>M. 10 hours after induction, the glowing cells were measured for spectrum using SHIMADZU RF5301PC Spectrofluorophotometer.</p> |
</div> | </div> | ||
<p>The time course of luxbrick expression and blue light emission under L-arabinose induction was given in a more visualized way, which is a short movie. As shown in the movie, the cells transformed with luxbrick begin to glow 9 hours after induction, and the entire visible glowing process lasts about 10 hours.</p> | <p>The time course of luxbrick expression and blue light emission under L-arabinose induction was given in a more visualized way, which is a short movie. As shown in the movie, the cells transformed with luxbrick begin to glow 9 hours after induction, and the entire visible glowing process lasts about 10 hours.</p> | ||
| Line 55: | Line 61: | ||
<p class="description">Movie1. the time course of our light emitting cell</p> | <p class="description">Movie1. the time course of our light emitting cell</p> | ||
</div> | </div> | ||
| - | |||
</div> | </div> | ||
<div class="PKU_context floatR" style="padding:12px 12px 13px 13px;"> | <div class="PKU_context floatR" style="padding:12px 12px 13px 13px;"> | ||
</html><groupparts>iGEM012 Peking</groupparts><html></div></html>{{Template:Peking2012_Color_Epilogue}} | </html><groupparts>iGEM012 Peking</groupparts><html></div></html>{{Template:Peking2012_Color_Epilogue}} | ||
Latest revision as of 14:35, 20 October 2012
Data for Favorite Parts
An Constitutive LuxBrick Generator(Main Page-BBa_K819005)
Testing device for the Luminesensor (Main Page-BBa_K819006) and measurement device for the Luminesensor (Main Page-BBa_K819007) a fast degrading GFP ligated to the downstream of SulA408 promoter and RecA408 promoter, respectively. These two promoters are regulated by the Luminesensor and works independently of the host genetic context.
Data for Optimized Parts

Figure 2. BL21 cells harboring T7-lux operon(BBa_K819008) are emitting blue luminescence under IPTG induction at 5x10-5M
Lux Operon under T7 promoter (Main Page-BBa_K819008)
We successfully improved the easy-of-use of “lux brick” (BBa_K325909) part constructed by Cambridge 2010 iGEM team by placing the coding sequence of luxbrick (luxCDABEG) and related RBS under T7 promoter (BBa_I712074).
T7 promoter separates sensing/circuitry functions from pathways/actuation. It is encoded in genetically distinct regions while linked with other circuits by providing the output for the circuits that drive the expression of phage polymerases. Luxbrick under T7 promoter is very modular, because it is transcribed by T7 polymerase, which can be placed under any other promoter, forming a interface between luxbrick and other systems.
Once transformed into BL21(de3)strain, high protein production can be achieved rapidly after IPTG induction.Final concentration of IPTG should be round 0.05mM.
To note that the incubating temperature should be no higher than 30oC, or the heavy Lux complex can easily aggregate.
Optimum incubating conditions provided by Peking iGEM 2012:
250 rpm, 22oC, good ventilation after induction(final concentration of IPTG: round 0.5 mM).
Data for Other Submitted Parts
Luminesensor repressible SulA408 promoter (Main Page-BBa_K819017)
Luminesensor repressible RecA408 promoter (Main Page-BBa_K819002)
These are the two promoters used in this year’s project to test the functionality of Luminesensor and measure the efficiency of it.
Weak CheZ generator (Main Page-BBa_K819009)
medium CheZ generator (Main Page-BBa_K819010)
Chemotaxis protein CheZ ligated to 2 constitutive promoters with varying strengths. These two parts express the CheZ protein in varied quantities, which corresponds to the different levels of induced mobility in the ΔCheZ E.coli strain.
Luminesensor repressible RecA408 promoter (Main Page-BBa_K819015)
Luminesensor repressible SulA408 promoter (Main Page-BBa_K819016)
Chemotaxis protein CheZ ligated to the Luminesensor repressible SulA408 promoter and RecA408 promoter, respectively. Presumably these two parts can would convert chemotaxis pathway of E.coli into a phototaxis pathway by controlling the expression level of CheZ in ΔCheZ strain in response to blue light.
Luminesensor repressible LacIM generator (Main Page-BBa_K819012)
Coding sequence of LacI mutant protein driven by the Luminesensor repressible SulA408 promoter (BBa_K819017).
Data for Pre-Existing Parts
luxbrick (Main Page-BBa_K325909)
luxbrick is a bacteria luciferase part constructed by Cambridge 2010 iGEM team. They successfully expressed bacterial luciferase in E.coli, which could produce blue light under L-arabinose induction, and they carefully characterized this part. But because there are data we need while they didn’t give, we made the following supplementary characterization.
The supplementary characterization and our application of luxbrick part were added to the experience page of this part (see Experience-BBa_K325909)
The spectrum of the light emitted from E.coli harboring luxbrick was measured and it showed a maximum intensity at 485nm.
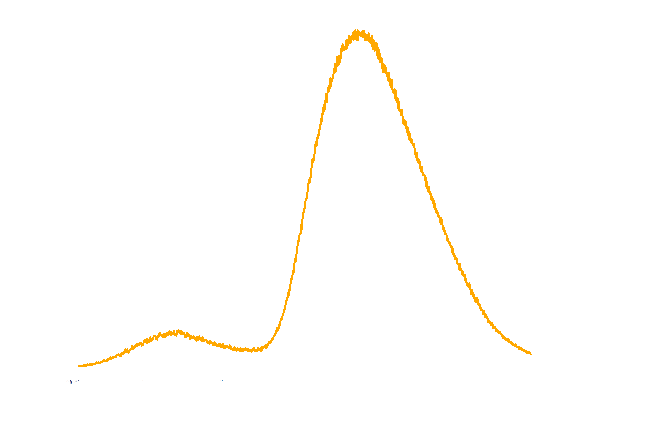
Figure 3. TOP10 cells harboring the luxbrick were cultured in LB medium and induced with L-arabinose at 10-3M. 10 hours after induction, the glowing cells were measured for spectrum using SHIMADZU RF5301PC Spectrofluorophotometer.
The time course of luxbrick expression and blue light emission under L-arabinose induction was given in a more visualized way, which is a short movie. As shown in the movie, the cells transformed with luxbrick begin to glow 9 hours after induction, and the entire visible glowing process lasts about 10 hours.
if you can not view this video,please click HERE.
Movie1. the time course of our light emitting cell
 "
"














