Team:Peking
From 2012.igem.org
Spring zhq (Talk | contribs) |
Spring zhq (Talk | contribs) |
||
| Line 859: | Line 859: | ||
<div class="floatR"><img src="/wiki/images/4/4f/Peking2012_LuminesensorMechanism.jpg" alt="" style="width:200px;"/></div> | <div class="floatR"><img src="/wiki/images/4/4f/Peking2012_LuminesensorMechanism.jpg" alt="" style="width:200px;"/></div> | ||
<div class="floatC"><p class="context_block" style="width:360px;"> | <div class="floatC"><p class="context_block" style="width:360px;"> | ||
| - | With strong motivation to raise a new generation of optogenetics, Peking iGEM team has rationally constructed a hypersensitive sensor of luminance – what we call the <i>Luminesensor</i>, with which spatiotemporal control of biochemical process or cellular behavior is highly feasible. It provides a paradigm for rational design of | + | With strong motivation to raise a new generation of optogenetics, Peking iGEM team has rationally constructed a hypersensitive sensor of luminance – what we call the <i>Luminesensor</i>, with which spatiotemporal control of biochemical process or cellular behavior is highly feasible. It provides a paradigm for rational design of sensor. |
</p></div> | </p></div> | ||
</div> | </div> | ||
Revision as of 14:31, 19 September 2012
Abstract
Optogenetic tools have made significant impact on life sciences and beyond. However, several serious issues remain: cytotoxicy, narrow dynamic range, and dependency on laser and exogenous chromophore. To circumvent these, Peking iGEM has rationally constructed a hypersensitive sensor of luminance -- Luminesensor. Primarily, the sensor was designed by fusing blue-light-sensing protein domain from Neurospora with DNA binding domain of LexA from E. coli, following which protein structure inspection and kinetic simulation were conducted to rationally perform optimization. Amazingly, Luminesensor was proved to be as sensitive as to sense natural light and even bioluminescence. With this sensor, spatiotemporal control of cellular behavior, such as phototaxis, high-resolution 2D and 3D bio-printing using dim light and even luminescence of iPad were shown to be very easy. What’s more, we successfully implemented cell-cell signaling using light, which is the very first time in synthetic biology and of great importance for biotechnological use.
Luminesensor
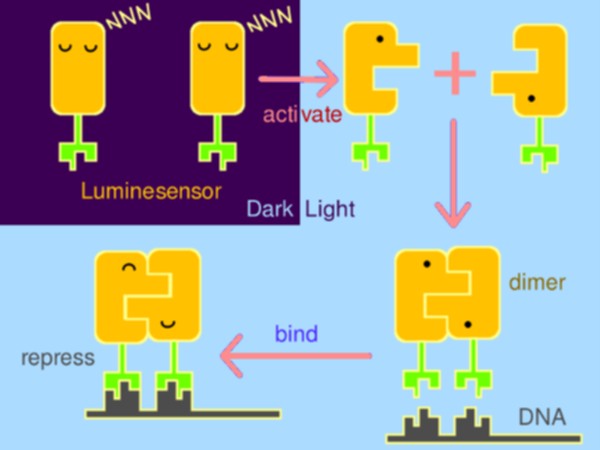
With strong motivation to raise a new generation of optogenetics, Peking iGEM team has rationally constructed a hypersensitive sensor of luminance – what we call the Luminesensor, with which spatiotemporal control of biochemical process or cellular behavior is highly feasible. It provides a paradigm for rational design of sensor.
Light Communication

The ultrasensitive Luminesensor is able to respond to very dim light and still maintain a high dynamic range. That encouraged Peking iGEM to explore the possibility of cell-cell communication through light. We have successfully implemented that , for the very first time, light-communication among cells without direct physical contact.
Syn Bio in 2D & 3D

3D printing is a new technology that has been rising for many years. The methods for 3D printing in manufacturing are quite developed, but in the realm of synthetic biology, there have been only a few attempts made. We believe that 3D printing can be utilized in many applications in both medical and manufacturing, such as with the synthesis of artificial vessels, organs, or bone tissues, and the creation of high-order biomaterials based on the high spatial resolution of the light. With more precise control of the response threshold of the optic biosensor, we can obtain holographic images from a solid medium.
Phototaxis
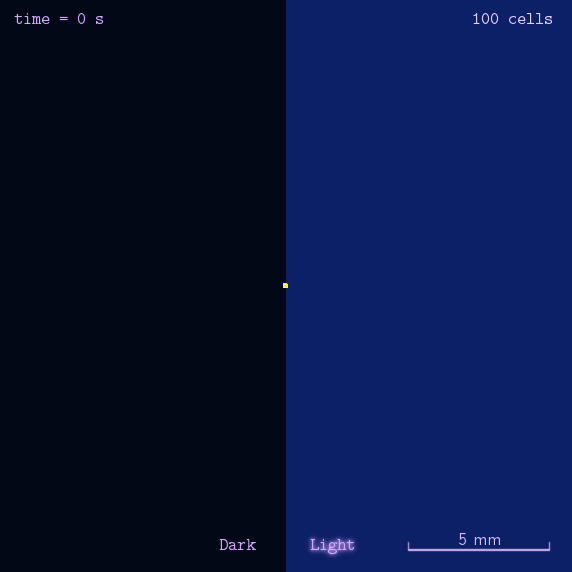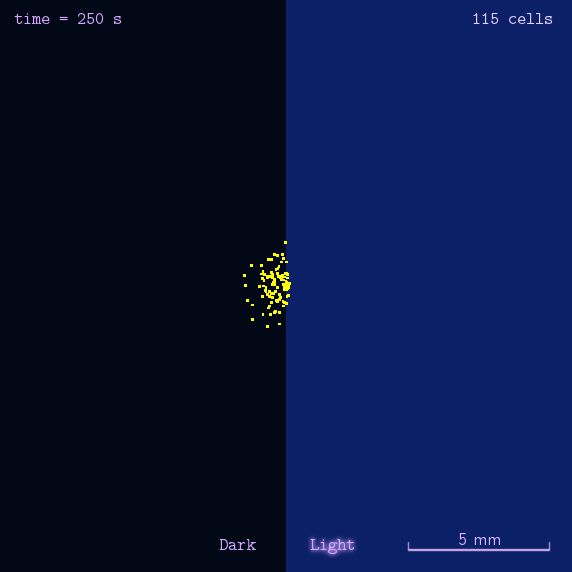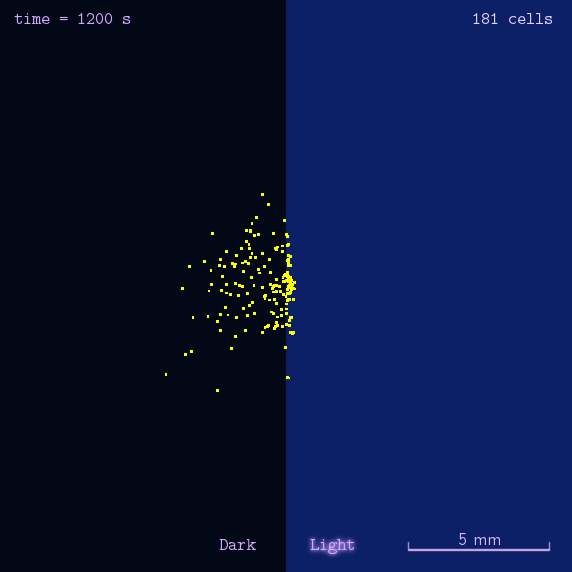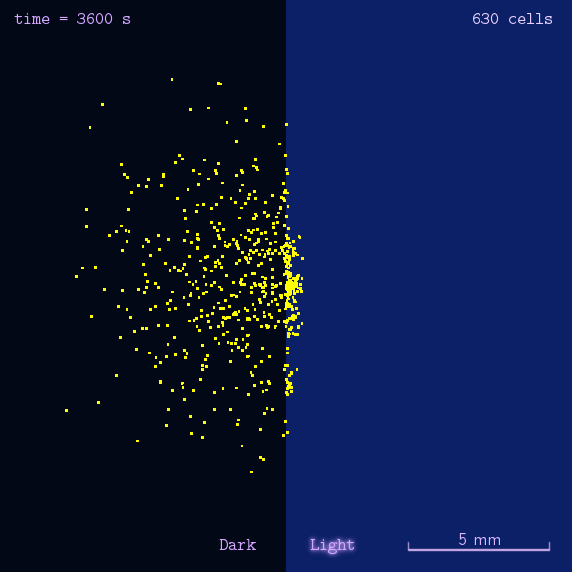
The 2012 Peking iGEM has successfully built "Phototatic" bacteria by programming the chemotaxis system in E. coli through light with the Luminesensor. By controlling the expression level of the cheZ protein with light, the tumbling frequency is coupled to the intensity of light signals. On the border of the light and dark fields on the plate, the motility difference of the cells in a single colony on the two sides is sufficient to form an uneven colony. The light-controlled cell motion that we have achieved has a very promising environmental and medical application for the future, e.g. the delivery of drugs to target concerns.
 "
"










