Team:NTU-Taida/Result/Penetration
From 2012.igem.org
Penetration Assay
Contents |
Method
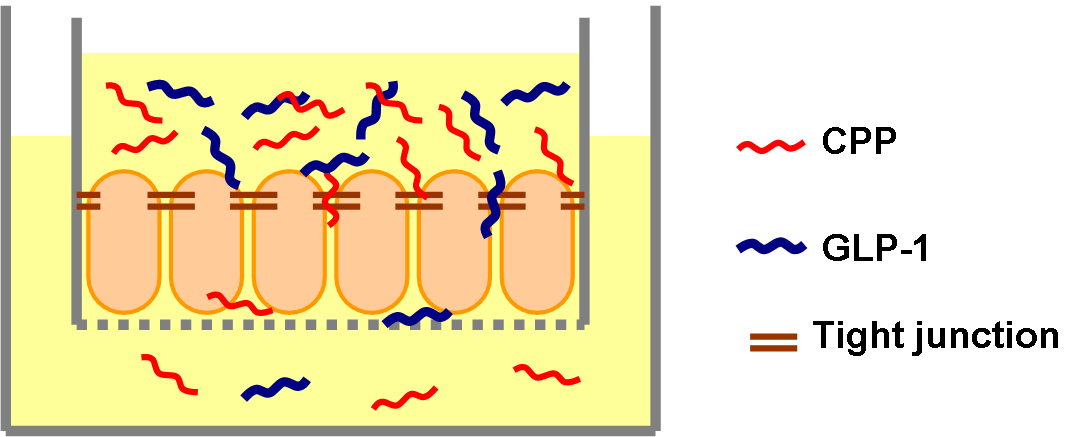
Previous studies have shown that Penetratin can help GLP-1 to increase its penetration rate to 5% in vivo.[1] We used similar transwell penetration assay method done by others before.[2] We seeded the intestinal cells in transwells and incubated for two weeks to let them grow to form tight junctions. The transwells were put on 24 well plates and were soaked in media (DMEM). We made 10 nm GLP-1 solution mixed with different concentration of Penetratin (10 nm, 50 nm, 100 nm, 500 nm) and added it to the upper portion of transwells. After different time (1 hr, 3 hr, 5 hr, 7 hr, 9 hr) we collected small amount of media in 6 wells (the lower portion of transwells) and measured the GLP-1 concentration by ELISA. We expected to see both time course and Penetratin dose-dependent manner of GLP-1 penetration.
Protocol
A. Transwell Penetration Assay
- Seed Caco-2 intestinal epithelial cells on transwells, and incubate them until the resistance is greater than 1000 Ohm/cm2 (Suggesting the cells are polarized and the tight junctions are well-formed[3]).
- Wash the transwells with PBS for 3 times, transfer the transwells to a new 6 well plate. There are 2 mL media (DMEM) in each well of the new plate.
- Add 500 μL 20 nM GLP-1 (in DMEM) to each transwell, and add 500 μL Penetratin (in DMEM) in ascending orders of concentration (20nm, 100 nm, 200 nm, 1000 nm). Start to keep time.
- Collect 100 μL media in the lower wells at 1 hr, 3 hr, 5 hr, 7 hr, 9 hr, and keep the sample in -20℃ before ELISA.
B. ELISA
- Wash the wells with 250 μL Wash Buffer.
- Add 100 μL Assay Buffer to each well, and add 100 μL standards in ascending orders to wells, and add samples in the remaining wells.
- Incubate at 4℃ overnight.
- Decant liquid from plate.
- Wash 5 times with 250 μL Wash Buffer.
- Add 200 μL Detection Conjugate in each well. Incubate 2 hr at room temperature.
- Wash 3 times with 250 μL Wash Buffer.
- Add 200 μL diluted substrate and incubate for 20 min.
- Read plate on fluorescence plate reader with excitation/emission wavelength of 355 nm/460 nm.
Data
Unfortunately, our data didn’t show that Penetratin helps to increase the penetration rate of GLP-1. We detected the GLP-1 concentration inside the lower wells, and GLP-1 concentration with Penetratin addition is just as same as the one without Penetration addition (only GLP-1 inside transwell), suggesting that in this case Penetratin didn’t help GLP-1 penetrate through intestinal epithelial barrier.
Discussion and Prospect
Previous studies showed that the device is supposed to work, but in our case it didn’t function well. Possible reason may be
- We didn’t use the right cell line.
- The GLP-1 and Penetratin combinatory concentration needs more optimization.
- Penetratin helped GLP-1 go into the intestinal epithelia but it cannot come out from epithelial cells.
Unfortunately we are not able to finish good data before wiki freeze, but we’ll keep trying before jamboree.
Reference
- Khafagy el-S, et al. (2009) Efficiency of cell-penetrating peptides on the nasal and intestinal absorption of therapeutic peptides and proteins. Int J Pharm 381(1):49-55
- Liang JF, et al. (2005) Insulin-cell penetrating peptide hybrids with improved intestinal absorption efficiency. Biochem Biophys Res Commun 335(3):734-8
- McCall IC, et al. (2009) Effects of phenol on barrier function of a human intestinal epithelial cell line correlate with altered tight junction protein localization. Toxicol Appl Pharmacol 241(1):61-70
 "
"