Team:Michigan/Project
From 2012.igem.org
(Difference between revisions)
| Line 5: | Line 5: | ||
<div id="basic"> | <div id="basic"> | ||
<h3>Abstract</h3> | <h3>Abstract</h3> | ||
| - | + | Recombinases can be used to create responsive, low background, boolean genetic circuit in biological systems. Further, it is theoretically possible to create complex control circuits using combinations of invertible DNA sequences. We utilized HbiF to augment an existing recombinase system in <i>Escherichia coli</i> that relied on FimE. A burst of induced, low level expression of one recombinase will invert the promoter flanked by the recombinase binding sites IRR and IRL, triggering a switch from strong expression of one to another set of proteins made downstream. Induced expression of the second recombinase will revert the promoter to its original orientation, triggering the original set of protein expression. The inversion will be sustained across cell divisions with little leaky protein expression and negligible performance degradation after repeated inversions. This is a heritable, binary memory system and can be used as a component in more complex systems. | |
<br><br> | <br><br> | ||
| Line 14: | Line 14: | ||
<h2>fimS or synthetic “flip” sequence </h2> | <h2>fimS or synthetic “flip” sequence </h2> | ||
| - | + | ||
The process of fimbriation in Escherichia coli is regulated by the enzyme-catalyzed inversion of a segment of DNA, often labeled fimS, by a series of tyrosine recombinases of which FimE and FimB are the most known. In the absence of ATP or external prosthetic groups, the fim recombinases introduce strand breaks in repeating regions flanking the fimS regulatory sequence and physically reverse the sequence, activating or repressing the expression of fimbriation proteins based on the orientation of a contained promoter. | The process of fimbriation in Escherichia coli is regulated by the enzyme-catalyzed inversion of a segment of DNA, often labeled fimS, by a series of tyrosine recombinases of which FimE and FimB are the most known. In the absence of ATP or external prosthetic groups, the fim recombinases introduce strand breaks in repeating regions flanking the fimS regulatory sequence and physically reverse the sequence, activating or repressing the expression of fimbriation proteins based on the orientation of a contained promoter. | ||
| - | < | + | <br> |
| + | |||
| - | |||
Inversion catalyzed by FimE was shown to be primarily unidirectional, inverting fimS from the “on” to “off” orientation(in regards to fimbriae production); the strong orientation bias stands in contrast to FimB, which is bidirectional inverting fimS both “off” to “on” and “on” to “off” generally forming an equilibrium between “off” and “on” states. # An inducible genetic circuit utilizing this property was engineered by Ham et al. possessing tightly controllable, strong protein expression with relatively low basal expression and a unique mode of activation, requiring only “pulses” of inducer rather than extended exposure. | Inversion catalyzed by FimE was shown to be primarily unidirectional, inverting fimS from the “on” to “off” orientation(in regards to fimbriae production); the strong orientation bias stands in contrast to FimB, which is bidirectional inverting fimS both “off” to “on” and “on” to “off” generally forming an equilibrium between “off” and “on” states. # An inducible genetic circuit utilizing this property was engineered by Ham et al. possessing tightly controllable, strong protein expression with relatively low basal expression and a unique mode of activation, requiring only “pulses” of inducer rather than extended exposure. | ||
| - | < | + | <br> |
| + | |||
| - | |||
1.M S McClain et all. “Roles of fimB and fimE in site-specific DNA inversion associated with phase variation of type 1 fimbriae in Escherichia coli.” J. Bacteriol. September 1991vol. 173 no. 17 5308-5314 | 1.M S McClain et all. “Roles of fimB and fimE in site-specific DNA inversion associated with phase variation of type 1 fimbriae in Escherichia coli.” J. Bacteriol. September 1991vol. 173 no. 17 5308-5314 | ||
| - | + | ||
<h2> | <h2> | ||
| Line 30: | Line 30: | ||
</h2> | </h2> | ||
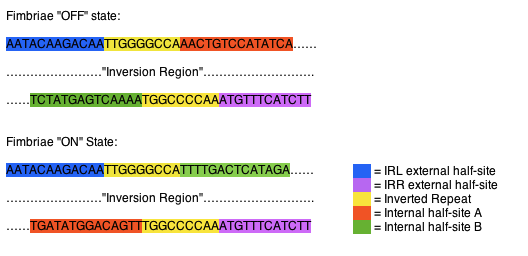
| - | + | The fimS region is composed of two inverted repeats, frequently referred to as inverted repeat left (IRL) and inverted repeat right (IRR), the two flank a region of DNA, referred to as the inversion region. Each of the inverted repeats are composed of three sections of DNA, an external half-site, an inverted repeat, and a internal half-site. The external half-sites and inverted repeats are stable and identifiers of the IRL and IRR, while the internal half-sites are mobile, switching between the IRL and IRR, dictating recombinase specificity and the orientation of the invertible region. The external half-sites are localized on the external sides of the fimS region, while the internal half-sites are both located directly adjacent to the inversion region and due to their location relative to the inverted repeats are inverted along with the inversion region when the region is acted upon by one of the recombinases (Fig 1). (1) | |
| - | The fimS region is composed of two inverted repeats, frequently referred to as inverted repeat left (IRL) and inverted repeat right (IRR), the two flank a region of DNA, referred to as the inversion region. Each of the inverted repeats are composed of three sections of DNA, an external half-site, an inverted repeat, and a internal half-site. The external half-sites and inverted repeats are stable and identifiers of the IRL and IRR, while the internal half-sites are mobile, switching between the IRL and IRR, dictating recombinase specificity and the orientation of the invertible region. The external half-sites are localized on the external sides of the fimS region, while the internal half-sites are both located directly adjacent to the inversion region and due to their location relative to the inverted repeats are inverted along with the inversion region when the region is acted upon by one of the recombinases (Fig 1). (1) | + | |
</html> | </html> | ||
| Line 46: | Line 45: | ||
<h2>FimE</h2> | <h2>FimE</h2> | ||
| - | + | ||
The FimE enzyme was cloned using the Caltech-made part K137007. A well characterized tyrosine recombinase, the enzyme catalyzes the inversion of a 200-300bp DNA region such that a 3’-facing sequence on the template strand of the region becomes a 3’-facing sequence on the coding strand. This functionality occurs in the lack of general cofactors or ATP, as demonstrated by Ham et al, possesses remarkable catalytic efficiency, with only very low levels capable of flipping regions efficiently. | The FimE enzyme was cloned using the Caltech-made part K137007. A well characterized tyrosine recombinase, the enzyme catalyzes the inversion of a 200-300bp DNA region such that a 3’-facing sequence on the template strand of the region becomes a 3’-facing sequence on the coding strand. This functionality occurs in the lack of general cofactors or ATP, as demonstrated by Ham et al, possesses remarkable catalytic efficiency, with only very low levels capable of flipping regions efficiently. | ||
| - | < | + | <br> |
| - | |||
FimE is dependent on properly oriented inverted repeats (IRs) flanking a region of interest for the reaction to occur. Furthermore, it may only invert a fim sequence in the “on” orientation to the “off” orientation. | FimE is dependent on properly oriented inverted repeats (IRs) flanking a region of interest for the reaction to occur. Furthermore, it may only invert a fim sequence in the “on” orientation to the “off” orientation. | ||
| - | + | ||
<h2>HbiF</h2> | <h2>HbiF</h2> | ||
| - | + | ||
HbiF, a tyrosine recombinase bearing high sequence homology to FimE and other fim recombinases, was discovered in 2006 by Xie et all and also catalyses inversion of the fimS region. This novel protein was harnessed to convert the FimE-activatable expression system described by Ham et al. into a bidirectional switch capable of binary states dependent on the presence and expression of either FimE or HbiF recombinases. Team Michigan’s submission of HbiF, K880000, possesses a protein fusion standard RFC25 suffix to facilitate circuit regulation or protein quantification. | HbiF, a tyrosine recombinase bearing high sequence homology to FimE and other fim recombinases, was discovered in 2006 by Xie et all and also catalyses inversion of the fimS region. This novel protein was harnessed to convert the FimE-activatable expression system described by Ham et al. into a bidirectional switch capable of binary states dependent on the presence and expression of either FimE or HbiF recombinases. Team Michigan’s submission of HbiF, K880000, possesses a protein fusion standard RFC25 suffix to facilitate circuit regulation or protein quantification. | ||
| - | < | + | <br> |
| + | |||
| - | |||
Opposite of FimE, HbiF catalyzes the rotation of fimS from the “off” to “on” position in vivo. | Opposite of FimE, HbiF catalyzes the rotation of fimS from the “off” to “on” position in vivo. | ||
| - | < | + | <br> |
| + | |||
| - | |||
Xie et all. “HbiF Regulates Type 1 Fimbriation Independently of FimB and FimE.” Infection and Immunity July 2006 vol. 74 No. 7 4039-4047. | Xie et all. “HbiF Regulates Type 1 Fimbriation Independently of FimB and FimE.” Infection and Immunity July 2006 vol. 74 No. 7 4039-4047. | ||
| - | + | ||
<h2>Further Reading</h2> | <h2>Further Reading</h2> | ||
| - | + | ||
Ham TS, Lee SK, Keasling JD, Arkin AP (2008) Design and Construction of a Double Inversion Recombination Switch for Heritable Sequential Genetic Memory. PLoS ONE 3(7): e2815. doi:10.1371/journal.pone.0002815 | Ham TS, Lee SK, Keasling JD, Arkin AP (2008) Design and Construction of a Double Inversion Recombination Switch for Heritable Sequential Genetic Memory. PLoS ONE 3(7): e2815. doi:10.1371/journal.pone.0002815 | ||
| - | |||
| - | < | + | |
| + | <br> | ||
Ham TS, Lee SK, Keasling JD, Arkin AP (2006) A Tightly Regulated Inducible Expression System Utilizing the fim Inversion Recombination Switch. Biotechnol. Bioeng., Vol. 94(1) doi:10.1002/bit.20916 | Ham TS, Lee SK, Keasling JD, Arkin AP (2006) A Tightly Regulated Inducible Expression System Utilizing the fim Inversion Recombination Switch. Biotechnol. Bioeng., Vol. 94(1) doi:10.1002/bit.20916 | ||
| - | + | ||
<br><br> | <br><br> | ||
<h3>Rationale</h3> | <h3>Rationale</h3> | ||
<h2>A Tightly-controlled, stable switch</h2> | <h2>A Tightly-controlled, stable switch</h2> | ||
| - | + | ||
| - | A system utilizing both FimE and HbiF has several advantages beyond established multi-state biological circuits. As the binding site for HbiF is the FimE binding site after inversion, the system contains a remarkable number of reusable components, and where a recombinase can only invert the switching region after the binding site is restored by the other recombinase. This limitation posed to the recombinases allow the host to remember its previous state as the inversion region is flipped, and will not respond to duplicates of the same signal. The switching sequence can also theoretically flip back and forth without generating new component and without being depleted. | + | A system utilizing both FimE and HbiF has several advantages beyond established multi-state biological circuits. As the binding site for HbiF is the FimE binding site after inversion, the system contains a remarkable number of reusable components, and where a recombinase can only invert the switching region after the binding site is restored by the other recombinase. This limitation posed to the recombinases allow the host to remember its previous state as the inversion region is flipped, and will not respond to duplicates of the same signal. The switching sequence can also theoretically flip back and forth without generating new component and without being depleted. |
<h2>Cheaper Biomanufacturing </h2> | <h2>Cheaper Biomanufacturing </h2> | ||
| - | + | ||
| - | A system utilizing both FimE and HbiF as actuators would allow for the creation of a genetic circuit that works in a manner analogous to a push-button switch. Current expression systems predominantly rely on inducible promoters systems, such as pBAD and the lac promoter, requiring a constant administration of inducers making them more analogous to a switch that is actively being held in the “on” position. The constant administration of inducers, such as arabinose and lactose, make up a large portion of the operating costs of biomanufacturing. FimE and HbiF in concert with separate inducible promoters could be used as a push-button switch where a short exposure to one of the inducers would trigger recombinase expression resulting in inversion of the switch and a new set of protein expression. This would improve on the recombinase system described in Ham et al. as the switch could be readily inverted in either direction, while the Ham et al. system would require re-seeding with uninverted cells everytime protein expression must change. | + | A system utilizing both FimE and HbiF as actuators would allow for the creation of a genetic circuit that works in a manner analogous to a push-button switch. Current expression systems predominantly rely on inducible promoters systems, such as pBAD and the lac promoter, requiring a constant administration of inducers making them more analogous to a switch that is actively being held in the “on” position. The constant administration of inducers, such as arabinose and lactose, make up a large portion of the operating costs of biomanufacturing. FimE and HbiF in concert with separate inducible promoters could be used as a push-button switch where a short exposure to one of the inducers would trigger recombinase expression resulting in inversion of the switch and a new set of protein expression. This would improve on the recombinase system described in Ham et al. as the switch could be readily inverted in either direction, while the Ham et al. system would require re-seeding with uninverted cells everytime protein expression must change. |
</div> | </div> | ||
| Line 92: | Line 90: | ||
</div> | </div> | ||
| - | + | ||
</div> | </div> | ||
Revision as of 22:55, 1 October 2012
Abstract
Recombinases can be used to create responsive, low background, boolean genetic circuit in biological systems. Further, it is theoretically possible to create complex control circuits using combinations of invertible DNA sequences. We utilized HbiF to augment an existing recombinase system in Escherichia coli that relied on FimE. A burst of induced, low level expression of one recombinase will invert the promoter flanked by the recombinase binding sites IRR and IRL, triggering a switch from strong expression of one to another set of proteins made downstream. Induced expression of the second recombinase will revert the promoter to its original orientation, triggering the original set of protein expression. The inversion will be sustained across cell divisions with little leaky protein expression and negligible performance degradation after repeated inversions. This is a heritable, binary memory system and can be used as a component in more complex systems.Background
fimS or synthetic “flip” sequence
The process of fimbriation in Escherichia coli is regulated by the enzyme-catalyzed inversion of a segment of DNA, often labeled fimS, by a series of tyrosine recombinases of which FimE and FimB are the most known. In the absence of ATP or external prosthetic groups, the fim recombinases introduce strand breaks in repeating regions flanking the fimS regulatory sequence and physically reverse the sequence, activating or repressing the expression of fimbriation proteins based on the orientation of a contained promoter.Inversion catalyzed by FimE was shown to be primarily unidirectional, inverting fimS from the “on” to “off” orientation(in regards to fimbriae production); the strong orientation bias stands in contrast to FimB, which is bidirectional inverting fimS both “off” to “on” and “on” to “off” generally forming an equilibrium between “off” and “on” states. # An inducible genetic circuit utilizing this property was engineered by Ham et al. possessing tightly controllable, strong protein expression with relatively low basal expression and a unique mode of activation, requiring only “pulses” of inducer rather than extended exposure.
1.M S McClain et all. “Roles of fimB and fimE in site-specific DNA inversion associated with phase variation of type 1 fimbriae in Escherichia coli.” J. Bacteriol. September 1991vol. 173 no. 17 5308-5314
Orientation of IRL/IRR Components of fimS dictate enzyme specificity
The fimS region is composed of two inverted repeats, frequently referred to as inverted repeat left (IRL) and inverted repeat right (IRR), the two flank a region of DNA, referred to as the inversion region. Each of the inverted repeats are composed of three sections of DNA, an external half-site, an inverted repeat, and a internal half-site. The external half-sites and inverted repeats are stable and identifiers of the IRL and IRR, while the internal half-sites are mobile, switching between the IRL and IRR, dictating recombinase specificity and the orientation of the invertible region. The external half-sites are localized on the external sides of the fimS region, while the internal half-sites are both located directly adjacent to the inversion region and due to their location relative to the inverted repeats are inverted along with the inversion region when the region is acted upon by one of the recombinases (Fig 1). (1)
Fig 1. Orientation of fimS inverted repeats. In the “off “state the IRL contains internal half-site A (orange), while the IRR contains internal half-site B (green). When inverted to the “on” state the IRL contains internal half-site B (green), while the IRR contains internal half-site A (orange).
(1) McCuster et al., Molecular Microbiology (2008) 67(1), 171–187
FimE
The FimE enzyme was cloned using the Caltech-made part K137007. A well characterized tyrosine recombinase, the enzyme catalyzes the inversion of a 200-300bp DNA region such that a 3’-facing sequence on the template strand of the region becomes a 3’-facing sequence on the coding strand. This functionality occurs in the lack of general cofactors or ATP, as demonstrated by Ham et al, possesses remarkable catalytic efficiency, with only very low levels capable of flipping regions efficiently.FimE is dependent on properly oriented inverted repeats (IRs) flanking a region of interest for the reaction to occur. Furthermore, it may only invert a fim sequence in the “on” orientation to the “off” orientation.
HbiF
HbiF, a tyrosine recombinase bearing high sequence homology to FimE and other fim recombinases, was discovered in 2006 by Xie et all and also catalyses inversion of the fimS region. This novel protein was harnessed to convert the FimE-activatable expression system described by Ham et al. into a bidirectional switch capable of binary states dependent on the presence and expression of either FimE or HbiF recombinases. Team Michigan’s submission of HbiF, K880000, possesses a protein fusion standard RFC25 suffix to facilitate circuit regulation or protein quantification.Opposite of FimE, HbiF catalyzes the rotation of fimS from the “off” to “on” position in vivo.
Xie et all. “HbiF Regulates Type 1 Fimbriation Independently of FimB and FimE.” Infection and Immunity July 2006 vol. 74 No. 7 4039-4047.
Further Reading
Ham TS, Lee SK, Keasling JD, Arkin AP (2008) Design and Construction of a Double Inversion Recombination Switch for Heritable Sequential Genetic Memory. PLoS ONE 3(7): e2815. doi:10.1371/journal.pone.0002815Ham TS, Lee SK, Keasling JD, Arkin AP (2006) A Tightly Regulated Inducible Expression System Utilizing the fim Inversion Recombination Switch. Biotechnol. Bioeng., Vol. 94(1) doi:10.1002/bit.20916
 "
"