Team:Calgary/Project/DataPage
From 2012.igem.org
| Line 24: | Line 24: | ||
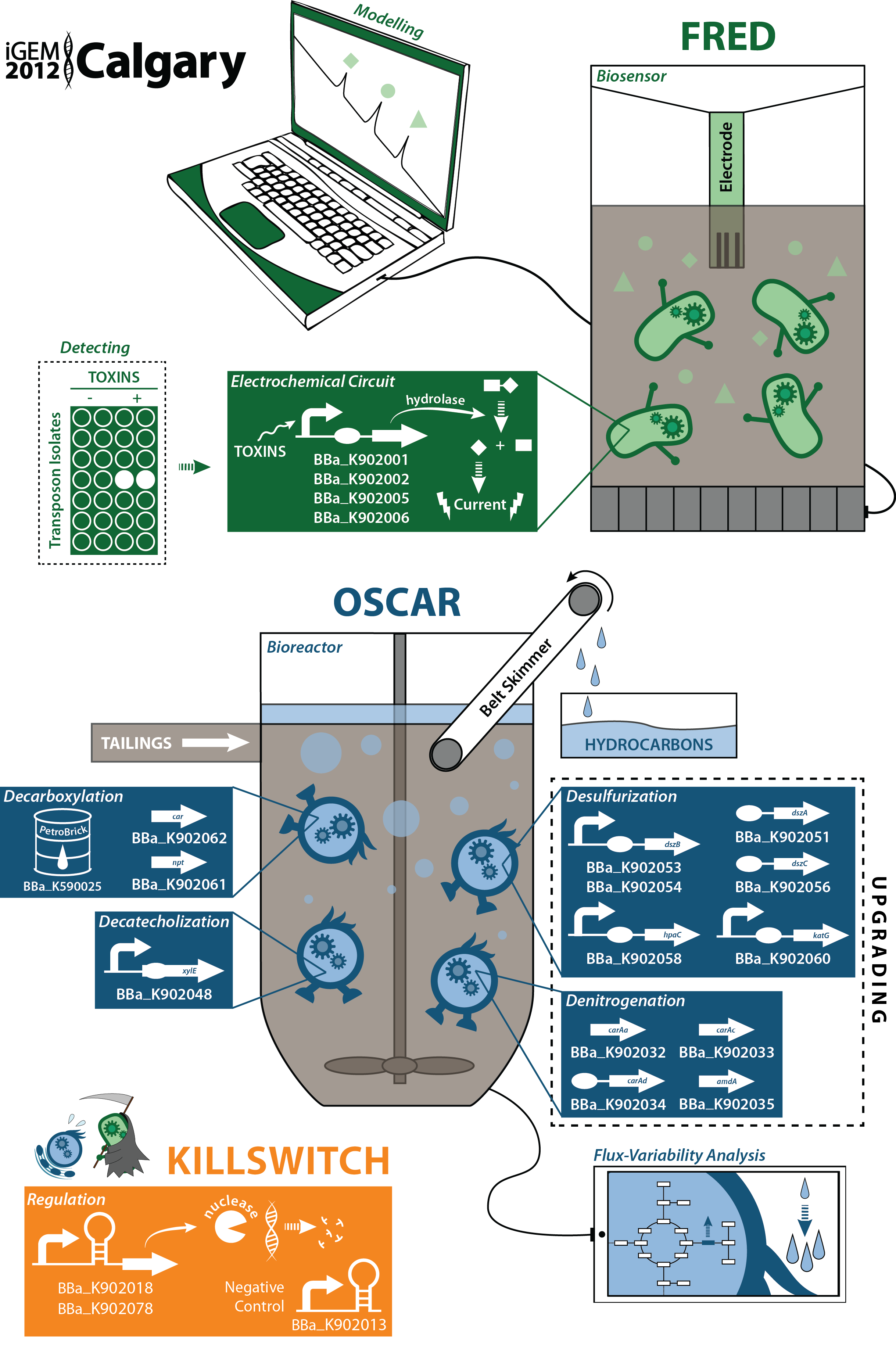
| - | </html>[[File:UCalgary2012_GraphicalAbstract.png|thumb|700px|center|thumb|Figure 1. The Calgary team has developed a dual system for the detection of toxic components in tailing ponds as well as the remediation of these compounds. Tailing ponds are large bodies of water containing waste products produced from the extraction of bitumen in the | + | </html>[[File:UCalgary2012_GraphicalAbstract.png|thumb|700px|center|thumb|Figure 1. The Calgary team has developed a dual system for the detection of toxic components in tailing ponds as well as the remediation of these compounds. Tailing ponds are large bodies of water containing waste products produced from the extraction of bitumen in the oil sands. Our biosensor involved the identification of a toxin promoter through a transposon screen. An electrochemical detector was implemented with a multiple output system allowing for the detection of multiple compounds simultaneously. This promoter/detector system was then complimented with the production of a biosensor prototype involving both a physical device and a software program for easy data analysis. |
Rather than just sensing toxins in the tailings ponds, our major objective were to detoxify the tailings through the reduction of toxins such as carboxylic acids (mainly naphthenic acids) and catechol, turning them into useable hydrocarbons. Purification of these hydrocarbons would contribute to an added economic and industrial benefit. In order to house this system, we also aimed to design a bioreactor for our bacteria as well as optimize product output through a flux-variability based model. Finally, in order to create higher quality hydrocarbons, we explored desulphurization and denitrogenation pathways in order to upgrade our fuel. To do this in a safe and environmentally sound way, we designed not only structural containment mechanisms, but also genetic containment mechanisms through novel inducible ribo-killswitches.]]<html> | Rather than just sensing toxins in the tailings ponds, our major objective were to detoxify the tailings through the reduction of toxins such as carboxylic acids (mainly naphthenic acids) and catechol, turning them into useable hydrocarbons. Purification of these hydrocarbons would contribute to an added economic and industrial benefit. In order to house this system, we also aimed to design a bioreactor for our bacteria as well as optimize product output through a flux-variability based model. Finally, in order to create higher quality hydrocarbons, we explored desulphurization and denitrogenation pathways in order to upgrade our fuel. To do this in a safe and environmentally sound way, we designed not only structural containment mechanisms, but also genetic containment mechanisms through novel inducible ribo-killswitches.]]<html> | ||
Revision as of 23:25, 3 October 2012


Hello! iGEM Calgary's wiki functions best with Javascript enabled, especially for mobile devices. We recommend that you enable Javascript on your device for the best wiki-viewing experience. Thanks!
Detect and Destroy: Data Page
Characterization of new parts submitted to the Registry
(BBa_K902000) and (BBa_K902004, ): two novel hydrolase enzymes were submitted to the registry for the hydrolysis of two different sugar conjugated electroactive compounds. Used in conjunction with the existing lacZ part in the registry (BBa_I732005), this allows for the electrochemical detection of three compounds with a single electrode. A uidA inducible generator (BBa_k902002,) was submitted and characterized electrochemically. This data can be found on our Electroreporting page.
(BBa_K902008),(BBa_K902023) and (BBa_K902074): three novel riboswitchs were submitted along with two associated promoters (BBa_K902008) and BBa_K902008) and an inducible/repressible promoter (BBa_K902065) were submitted to the registry. One of these riboswitches (BBa_K902008) was tested with GFP and a constitutive promoter using this construct, (BBa_K902021), with its promoter and GFP using this construct (BBa_K902017) and with its promoter and the S7 kill gene using this construct (BBa_K902018). This data can be found on our killswitch Regulation page.
Genes for denitrogenation and desulphurization were biobricked and submitted. A novel oxidoreductase part (BBa_K902058) was also submitted and characterized for use in the desulphurization project. This data can be found on our uprading Desulfurization page.
Further characterization of parts already present within the registry
(BBa_K590025), the PetroBrick, submitted by the Washington team in 2011, was characterized for a novel function: the conversion of naphthenic acids and 2-hydroxymuconate- a catechol break-down product from from the xylE gene (BBa_J33204) into hydrocarbons. This data can be found on both the decarboxylation page and the Decatecholization page.
The output of (BBa_K590025) was also optimized thorugh a program we devlopped in MATLAB for the optimization of metabolic pathways in synthetic biology metabolic networks. The program allows you to build an artificial synthetic biology network in E. coli and predicts substrates that should be fed to the organism to increase production of the compound. This was characterized and validated in the wetlab with the Petrobrick. This data can be found on our Flux Analysis page.
An existing xylE gene in the registry (BBa_J33204) was constructed with a constitutive promoter instead of the glucose-repressible one it is available with in the registry. This allows for increased output in media containing glucose, making it more suitable for a variety of applications such as our own. We validated the functionality of this part which can be found on our Catechol Degradation page.
The IPTG inducible lacI regulated promoter (BBa_R0010) was tested electorchemically todemonstarte its leakiness when not used in conjunction with strong expression of regulatory elements. This data can be found on our Electroreporting page.
Additional Work and Characterization
Designed and prototyped a physical bioreactor for which we obtained both qualitative and quantitaive data for its functionality. This is outlined on our Bioreactor page.
Developed and tested both hardware and software of a biosensor using an electrochemical sensor. The software is available on our wiki on our biosensor (Robert can you help me flush this out a bit, or one of the engineers?
Submitted novel parts involved in decarboxylation and validated an additional single gene (oleT) for functionality in its native host. This data can be found in our decarboxylation section, however this gene has not yet been submitted due to problems in cloning it.
Characterizedone of our constitutively expressed transposon clones to test the lacZ gene electrochemically. This data can be found on or electroreporting page.
 "
"