Team:Calgary/Project
From 2012.igem.org


Hello! iGEM Calgary's wiki functions best with Javascript enabled, especially for mobile devices. We recommend that you enable Javascript on your device for the best wiki-viewing experience. Thanks!
Project Overview
Toxins In Our Environment
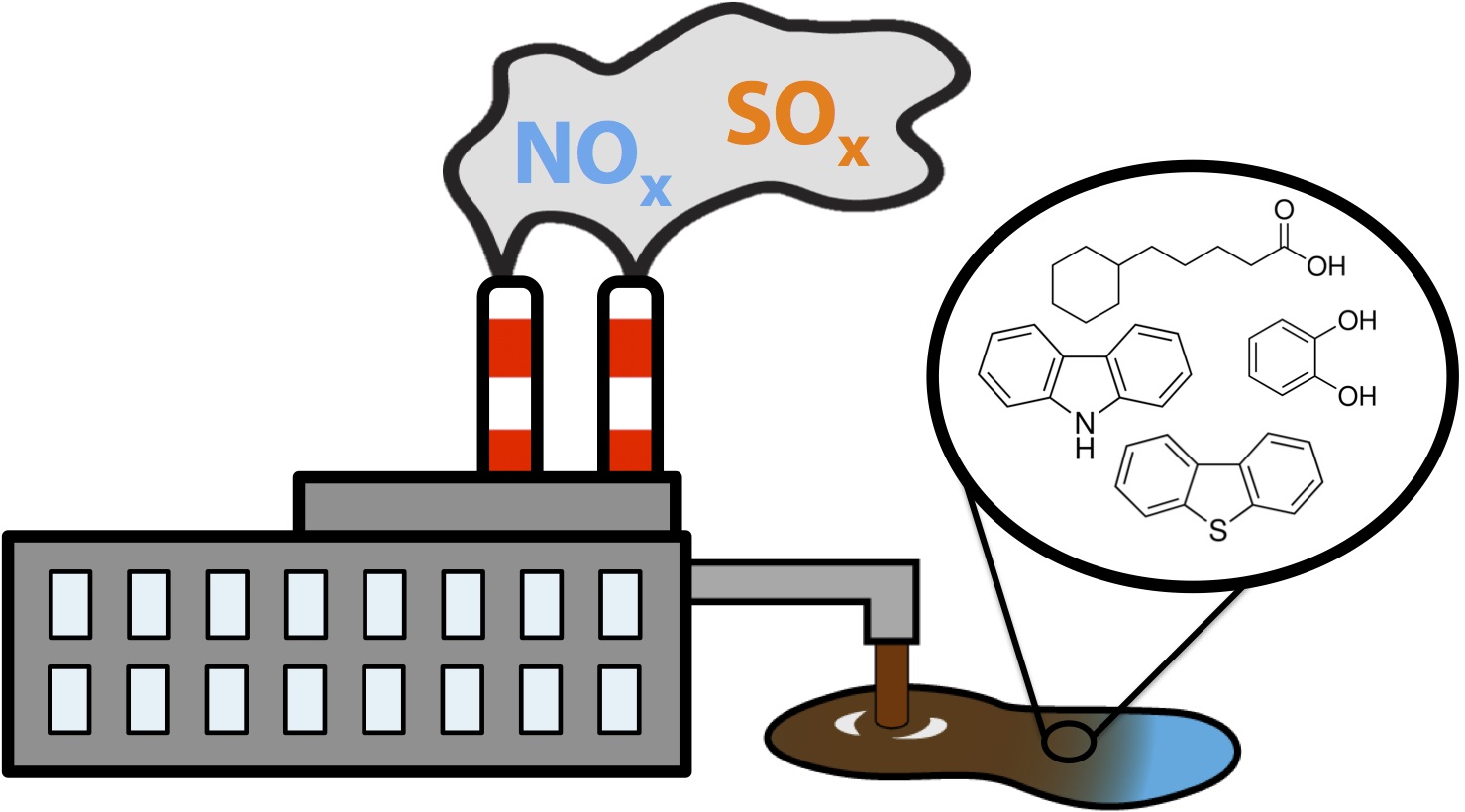
During the production of oil, gas, and many other natural products there is the production of numerous toxic components. These have become a huge problem in our society resulting in land, water, and air contamination. These consist of a variety different types of compounds. Air contaminants consist of NOx (nitrogen containing compounds) and SOx (sulfur containing compounds) which contribute to a variety of environmental issues including green house gas accumulation and acid rain. Similarly water contaminants often consist of complex mixtures of compounds including highly toxic phenols and aromatic compounds, corrosive and additionally toxic carboxylic acid containing compounds, sulfur, and nitrogen containing compounds. These often have complicated structures and demonstrate acute toxicity to wild life. Classical examples of water contamination include tailing ponds produced from the oil extraction process. Finally, land areas can become contaminated as a result of these toxic compounds leaching into ground water sources, spills or accidental release of waste products into the environment, and other ways.
Synthetic Biology As A Platform For Remediation
Presently, there are a variety of solutions to removing these compounds from the environment. This has been facilitated by stricter regulations from government bodies as well as better chemical and physical techniques for reducing the release of these compounds. Toxins which are released into the environment can be removed using various chemical agents or by physically removing contaminated soil or water areas. However there is still no efficient, environmentally healthy mechanism for this to occur. What needs to occur in order to better remediate these toxin's from the environment? We require a method to be able to easily detect where toxins are and also have a system for removing them from the environment. Microorganisms in the environment have evolved to be able to do both of these functions, responding to compounds in their environment and transform them into other products. Therefore we can harness these abilities to apply synthetic biology to these problems.
Introducing...
We would like to introduce FRED and OSCAR! Our dynamic biosensor/bioreactor duo designed to be able to detect toxic compounds such as the ones illustrated above in liquid waste and contaminated waters and also be able to convert these toxic components into useable hydrocarbons. FRED or the Functional Robust Electrochemical Detector, is capable of detecting various toxic components through an electrochemical response at the same time. We illustrated how this sensor could work by showing that it has the potential to detect toxins in contaminated water. Additionally, we developed a minaturized circuit for a prototype, validated that this device worked in the wetlab, and designed our own software available to everyone to be used with a home made potentiostat. OSCAR or the Optimized System for Carboxylic Acid Remediation is designed specifically to target naphthenic acids (NAs) the carboxylic acid containing compounds in the tailing ponds for their degradation. Using the Petrobrick (BBa______) we were able to convert various different naphthenic acid based compounds into their hydrocarbon analogs. Additionally, we wanted to be able to degrade other toxic components of tailings so we used the xylE gene in order to cleave catechol, an abundant intermediate in many toxic areas. Not only did we set out to break down catechol but we
 "
"