Team:Amsterdam/achievements/stochastic model
From 2012.igem.org
(Difference between revisions)
(→Leaky expression rate) |
(→References) |
||
| (22 intermediate revisions not shown) | |||
| Line 27: | Line 27: | ||
ordinary differnential equations are unsuited towards modelling systems with small integer amounts of the constituting species. | ordinary differnential equations are unsuited towards modelling systems with small integer amounts of the constituting species. | ||
In these cases a finer-grained modelling method is called for: the Stochastic Simulation Algorithm by Gillespie. | In these cases a finer-grained modelling method is called for: the Stochastic Simulation Algorithm by Gillespie. | ||
| - | By modelling each individual molecular reaction separately, the discreteness of this small amounts system is accounted for. | + | By modelling each individual molecular reaction separately, the discreteness of this small amounts system is accounted for. |
| + | Despite the small amounts of species present in the system analyzed here, we found no qualitative differences between the ODE and stochastic versions of the model. | ||
==== Comparison of SSA implementations ==== | ==== Comparison of SSA implementations ==== | ||
During the course of the summer we've investigated many stochastic simulation packages with implementation of the direct algorithms and some more coarse-grained optimizations thereof (e.g. the tau-leaping algorithm). | During the course of the summer we've investigated many stochastic simulation packages with implementation of the direct algorithms and some more coarse-grained optimizations thereof (e.g. the tau-leaping algorithm). | ||
| - | Most notable are [www.xlr8r.info/SSA/ xSSA] for Mathematica and [http://www.stompy.sourceforge.net StochPy] for Python. | + | Most notable are [http://www.xlr8r.info/SSA/ xSSA] for Mathematica and [http://www.stompy.sourceforge.net StochPy] for Python. |
The former seems a great tool, but at the time of writing seems to lack the ability to process third order reactions (reactions with three reactants). | The former seems a great tool, but at the time of writing seems to lack the ability to process third order reactions (reactions with three reactants). | ||
The latter has been developed at the Free University Amsterdam by Timo Maarleveld, currently PhD-student there. | The latter has been developed at the Free University Amsterdam by Timo Maarleveld, currently PhD-student there. | ||
In using this package, we've kept in close contact with Timo and submitted a lot of bug reports, helping the software to grow. | In using this package, we've kept in close contact with Timo and submitted a lot of bug reports, helping the software to grow. | ||
| - | Additionally, we've also extended StochPy by writing a supplementary tool [Team:Amsterdam/ | + | Additionally, we've also extended StochPy by writing a supplementary tool [https://2012.igem.org/Team:Amsterdam/extra/software MDL2LaTeX], that eases the publishing of models developed with StochPy. |
=Leakiness and background noise= | =Leakiness and background noise= | ||
| Line 51: | Line 52: | ||
==ODE Model definition== | ==ODE Model definition== | ||
| + | <table align="right"> | ||
| + | <tr><th>Parameter</th><th>Value</th></tr> | ||
| + | <tr><td>Ca</td> <td>$200\ \text{or}\ 40$</td> | ||
| + | <tr> | ||
| + | <td>ksFP</td> <td>$30$</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>lMFP</td> <td>$0.462$</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>lFP</td> <td>$0.2$</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td>kPlas</td> <td>$0.00866434 \cdot Ca$</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td> lPlas </td> <td> $0.00866434$ </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td colspan="2" align="center">Parameter values used to <br>plot the ODE-system</td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | |||
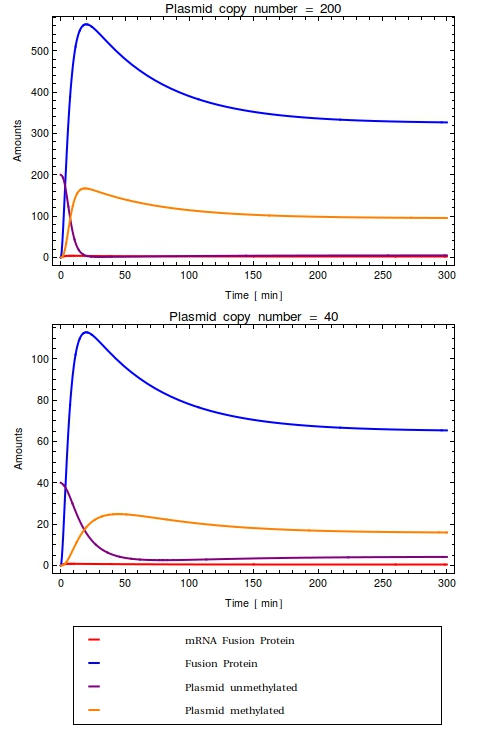
[[File:Combinedode.png|thumb|right|300px|Time trajectories of ODE-model in which the construct has been inserted in a high copy number plasmid (top, $Ca = 200$) and a low copy number plasmid (bottom, $Ca = 40$). All initial species values are set to $0$, except for the intial value of unmethylated plasmids which is equal to $Ca$. With these parameters, the dynamic range of the system is completely taken up by the background noise. No qualitative difference is observed between the high and low copy number cases]] | [[File:Combinedode.png|thumb|right|300px|Time trajectories of ODE-model in which the construct has been inserted in a high copy number plasmid (top, $Ca = 200$) and a low copy number plasmid (bottom, $Ca = 40$). All initial species values are set to $0$, except for the intial value of unmethylated plasmids which is equal to $Ca$. With these parameters, the dynamic range of the system is completely taken up by the background noise. No qualitative difference is observed between the high and low copy number cases]] | ||
| Line 73: | Line 97: | ||
\text{O} = \text{PlasU} + \text{PlasM} | \text{O} = \text{PlasU} + \text{PlasM} | ||
$$ | $$ | ||
| + | |||
| + | == Stochastic model definition == | ||
| + | |||
| + | The equations in the stochastic model are qualitatively equal to the ones in the differential equation model. | ||
| + | The equations, parameter and initial species values can be viewed on [[Team:Amsterdam/achievements/stochastic_model_definition|this page]]. | ||
| + | Analyzing the behaviour of this model by looking at the time-lapse plots, we see a similar trend as in the ODE-model: | ||
| + | all plasmids are methylated within a short amount of time. The fusion protein amounts are shown to be widely varying, but this does not alter the fraction of methylated plasmids much. | ||
| + | |||
| + | [[File:Stoch_single.png|thumb|200px|Single trajectory of stochastic model. Just as in the deterministic model, all plasmids are methylated within a short amount of time due to the leaky expression with the used values for $ k_{cat} $ and $ k_{cFP} $]] | ||
== Retrieving sensible parameter values == | == Retrieving sensible parameter values == | ||
| Line 94: | Line 127: | ||
More simply, the leaky expression rate can also be retrieved from this [[http://oregonstate.edu/instruction/bb492/lectures/Regulation.html|website]], which states a 1000-fold decrease in expression of the repressor-bound operon compared to the free operon. | More simply, the leaky expression rate can also be retrieved from this [[http://oregonstate.edu/instruction/bb492/lectures/Regulation.html|website]], which states a 1000-fold decrease in expression of the repressor-bound operon compared to the free operon. | ||
| - | == Fusion protein catalysis rate == | + | ==== Fusion protein catalysis rate ==== |
The catalysis rate $k_{cat}$ has not been determined for the MTase that we are using, M.ScaI. | The catalysis rate $k_{cat}$ has not been determined for the MTase that we are using, M.ScaI. | ||
However, for another MTase with the same [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=2.1.1.113 EC2.1.1.113 number] as M.ScaI, BamH1, the $k_{cat}$ has been determined to be $0.0175$ ([[#Cheng|1]]). | However, for another MTase with the same [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=2.1.1.113 EC2.1.1.113 number] as M.ScaI, BamH1, the $k_{cat}$ has been determined to be $0.0175$ ([[#Cheng|1]]). | ||
| - | == Cell growth rate == | + | ==== Cell growth rate ==== |
Our own experiments showed that the bacterial strain <math>DH5\alpha</math> transformed with two of our preliminary constructs had growth rates (<math>\mu</math>) between $80\ min^{-1}$ and $90\ min^{-1}$ ([Team:Amsterdam/Project/Growth_curves Experimental growth curves]). | Our own experiments showed that the bacterial strain <math>DH5\alpha</math> transformed with two of our preliminary constructs had growth rates (<math>\mu</math>) between $80\ min^{-1}$ and $90\ min^{-1}$ ([Team:Amsterdam/Project/Growth_curves Experimental growth curves]). | ||
In these simulations, cellular division was assumed to be either constant at 80 for single cells models or Gaussian distributed with $\mu = 80$ and $\sigma = 2$ in the cell division model. | In these simulations, cellular division was assumed to be either constant at 80 for single cells models or Gaussian distributed with $\mu = 80$ and $\sigma = 2$ in the cell division model. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
Looking at the plots for both the high and the low copy number plasmids, no qualitative difference is observable. All species in the system simply reach a steady state defined as the fraction between their production and degradation rates. | Looking at the plots for both the high and the low copy number plasmids, no qualitative difference is observable. All species in the system simply reach a steady state defined as the fraction between their production and degradation rates. | ||
Most notably, in the steady state that this system reaches all plasmids are methylated. | Most notably, in the steady state that this system reaches all plasmids are methylated. | ||
This is very undesirable of course! | This is very undesirable of course! | ||
| + | |||
| + | ==== Other parameters ==== | ||
| + | Values for the mRNA degradation rate and protein synthesis have been taking from ([[#Mantzaris|2]]). | ||
== Steady state parameter scanning == | == Steady state parameter scanning == | ||
| Line 136: | Line 149: | ||
Our experimental results can thus be explained by any combination of the two parameters which which has an intermediate value in plot. | Our experimental results can thus be explained by any combination of the two parameters which which has an intermediate value in plot. | ||
| - | + | A similar parameter scan has been painstakingly performed with the stochastic model. Simulations with 20 trajectories of 200-minute simulations for each parameter combination in a two-dimensional scan for both the $k_{cat}$ and $k_{cFP}$ in the same ranges as the ODE parm-scan. Unfortunately, even for these high amount of replications the variability in the resulting methylation fractions is very high, such that no trends are discernible at all in the results (data not shown). This variability can probably be reduced by performing even longer simulations and using more trajectories. A much more efficient implementation of the SSA will have to be used to reach this goal within reasonable time scales however. We hope to finish these simulations before the Jamboree. Because the deterministic simulations show similar qualitative behaviour as the more realistic stochastic simulations in the time-lapse plots, one could argue that we will obtain similar results here as the much less computationally expensive ODE-parameter scan. | |
| - | The | + | The steady state fraction of methylation shows almost no dependence upon the leaky transcription rate in the ODE model. This is likely caused by the steady state value that the mRNA reaches fairly quickly. The parameter scan density plots shows a very weak to no influence of the background noise. The fact that the degradation rate of the mRNA is much larger than the leaky transcription rate probably causes this. On the contrary, the steady state fraction of methylated plasmids is shown to be heavily dependent upon the catalysis rate of the fusion protein. This can steer future experiments to research the effects of lowered catalysis rates. |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
= Discussion = | = Discussion = | ||
| - | + | Unlike our initial expectations, the ODE-model analyzed here suggests that the catalysis rate of the FP is more important to the high background noise we have observed in the experiments than the leaky expression rate. | |
| - | + | This yields a new hypothesis to test in the lab: will lowering the catalysis rate significantly lower the background methylation rate? | |
| + | This raises the question on how to lower the catalysis rate of the MTase. A few methods to do this come to mind: | ||
| + | * Lower the temperature during the experiments, which will slow down all reactions in the cell including the catalysis rate | ||
| + | * Mutate amino acid sequence of binding domain, making the affinity of the for the DNA smaller | ||
| + | * Mutate some other part of the protein | ||
| + | * Pick a different methyltransferase, one that has a lower catalysis rate | ||
| + | * Attach a fluorescent protein to the methyltransferase | ||
=References= | =References= | ||
| Line 289: | Line 170: | ||
</span> | </span> | ||
| + | <span id="Mantzaris"> | ||
| + | <sup> | ||
| + | Stamatakis, M., & Mantzaris, N. V. (2009). Comparison of deterministic and stochastic models of the lac operon genetic network. Biophysical journal, 96(3), 887–906. doi:10.1016/j.bpj.2008.10.028 | ||
| + | </sup> | ||
| + | </span> | ||
</div> | </div> | ||
Latest revision as of 03:27, 27 September 2012
 "
"