|
|
| (8 intermediate revisions not shown) |
| Line 1: |
Line 1: |
| - | <html xmlns:v="urn:schemas-microsoft-com:vml"
| + | {{Template:Austin_Texas/Stylesheet}} |
| - | xmlns:o="urn:schemas-microsoft-com:office:office"
| + | |
| - | xmlns:w="urn:schemas-microsoft-com:office:word"
| + | |
| - | xmlns:m="http://schemas.microsoft.com/office/2004/12/omml"
| + | |
| - | xmlns="http://www.w3.org/TR/REC-html40">
| + | |
| | | | |
| - | <head> | + | <html> |
| - | <meta http-equiv=Content-Type content="text/html; charset=windows-1252">
| + | <ul class="cssmenu"> |
| - | <meta name=ProgId content=Word.Document> | + | <li class="home"><a href="/Team:Austin_Texas" title="home"><span class="displace">Home</span></a></li> |
| - | <meta name=Generator content="Microsoft Word 14">
| + | <li class="team"><a href="/Team:Austin_Texas/Team" title="team"><span class="displace">Team</span></a></li> |
| - | <meta name=Originator content="Microsoft Word 14"> | + | <li class="official_team_profile"><a href="https://igem.org/Team.cgi?year=2012&team_name=Austin_Texas" title="official_team_profile"><span class="displace">Official Team Profile</span></a></li> |
| - | <link rel=File-List href="p_files/filelist.xml"> | + | <li class="project"><a href="/Team:Austin_Texas/Project" title="project"><span class="displace">Project</span></a></li> |
| - | <!--[if gte mso 9]><xml>
| + | <li class="parts_submitted"><a href="/Team:Austin_Texas/Parts" title="parts_submitted"><span class="displace">Parts Submitted</span></a></li> |
| - | <o:DocumentProperties>
| + | <li class="modelling"><a href="/Team:Austin_Texas/Modeling" title="modeling"><span class="displace">Modeling</span></a></li> |
| - | <o:Author>Quandt, Erik M</o:Author>
| + | <li class="notebook"><a href="/Team:Austin_Texas/Notebook" class="selected" title="notebook"><span class="displace">Notebook</span></a></li> |
| - | <o:Template>Normal</o:Template>
| + | <li class="safety"><a href="/Team:Austin_Texas/Safety" title="safety"><span class="displace">Safety</span></a></li> |
| - | <o:LastAuthor>Quandt, Erik M</o:LastAuthor>
| + | <li class="attributions"><a href="/Team:Austin_Texas/Attributions" title="attributions"><span class="displace">Attributions</span></a></li> |
| - | <o:Revision>1</o:Revision>
| + | </ul> |
| - | <o:TotalTime>0</o:TotalTime>
| + | </html> |
| - | <o:Created>2012-08-20T17:46:00Z</o:Created>
| + | |
| - | <o:LastSaved>2012-08-20T17:46:00Z</o:LastSaved>
| + | |
| - | <o:Pages>5</o:Pages>
| + | |
| - | <o:Words>654</o:Words>
| + | |
| - | <o:Characters>3728</o:Characters>
| + | |
| - | <o:Company>University of Texas at Austin</o:Company>
| + | |
| - | <o:Lines>31</o:Lines>
| + | |
| - | <o:Paragraphs>8</o:Paragraphs>
| + | |
| - | <o:CharactersWithSpaces>4374</o:CharactersWithSpaces>
| + | |
| - | <o:Version>14.00</o:Version>
| + | |
| - | </o:DocumentProperties>
| + | |
| - | <o:OfficeDocumentSettings>
| + | |
| - | <o:AllowPNG/>
| + | |
| - | </o:OfficeDocumentSettings>
| + | |
| - | </xml><![endif]-->
| + | |
| - | <link rel=themeData href="p_files/themedata.thmx">
| + | |
| - | <link rel=colorSchemeMapping href="p_files/colorschememapping.xml">
| + | |
| - | <!--[if gte mso 9]><xml> | + | |
| - | <w:WordDocument>
| + | |
| - | <w:SpellingState>Clean</w:SpellingState>
| + | |
| - | <w:GrammarState>Clean</w:GrammarState>
| + | |
| - | <w:TrackMoves>false</w:TrackMoves>
| + | |
| - | <w:TrackFormatting/>
| + | |
| - | <w:PunctuationKerning/>
| + | |
| - | <w:ValidateAgainstSchemas/>
| + | |
| - | <w:SaveIfXMLInvalid>false</w:SaveIfXMLInvalid>
| + | |
| - | <w:IgnoreMixedContent>false</w:IgnoreMixedContent>
| + | |
| - | <w:AlwaysShowPlaceholderText>false</w:AlwaysShowPlaceholderText>
| + | |
| - | <w:DoNotPromoteQF/>
| + | |
| - | <w:LidThemeOther>EN-US</w:LidThemeOther>
| + | |
| - | <w:LidThemeAsian>X-NONE</w:LidThemeAsian>
| + | |
| - | <w:LidThemeComplexScript>X-NONE</w:LidThemeComplexScript>
| + | |
| - | <w:Compatibility>
| + | |
| - | <w:BreakWrappedTables/>
| + | |
| - | <w:SnapToGridInCell/>
| + | |
| - | <w:WrapTextWithPunct/>
| + | |
| - | <w:UseAsianBreakRules/>
| + | |
| - | <w:DontGrowAutofit/>
| + | |
| - | <w:SplitPgBreakAndParaMark/>
| + | |
| - | <w:EnableOpenTypeKerning/>
| + | |
| - | <w:DontFlipMirrorIndents/>
| + | |
| - | <w:OverrideTableStyleHps/>
| + | |
| - | </w:Compatibility>
| + | |
| - | <m:mathPr>
| + | |
| - | <m:mathFont m:val="Cambria Math"/>
| + | |
| - | <m:brkBin m:val="before"/>
| + | |
| - | <m:brkBinSub m:val="--"/>
| + | |
| - | <m:smallFrac m:val="off"/>
| + | |
| - | <m:dispDef/>
| + | |
| - | <m:lMargin m:val="0"/>
| + | |
| - | <m:rMargin m:val="0"/>
| + | |
| - | <m:defJc m:val="centerGroup"/>
| + | |
| - | <m:wrapIndent m:val="1440"/>
| + | |
| - | <m:intLim m:val="subSup"/>
| + | |
| - | <m:naryLim m:val="undOvr"/>
| + | |
| - | </m:mathPr></w:WordDocument>
| + | |
| - | </xml><![endif]--><!--[if gte mso 9]><xml> | + | |
| - | <w:LatentStyles DefLockedState="false" DefUnhideWhenUsed="true"
| + | |
| - | DefSemiHidden="true" DefQFormat="false" DefPriority="99"
| + | |
| - | LatentStyleCount="267">
| + | |
| - | <w:LsdException Locked="false" Priority="0" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" QFormat="true" Name="Normal"/>
| + | |
| - | <w:LsdException Locked="false" Priority="9" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" QFormat="true" Name="heading 1"/>
| + | |
| - | <w:LsdException Locked="false" Priority="9" QFormat="true" Name="heading 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="9" QFormat="true" Name="heading 3"/>
| + | |
| - | <w:LsdException Locked="false" Priority="9" QFormat="true" Name="heading 4"/>
| + | |
| - | <w:LsdException Locked="false" Priority="9" QFormat="true" Name="heading 5"/>
| + | |
| - | <w:LsdException Locked="false" Priority="9" QFormat="true" Name="heading 6"/>
| + | |
| - | <w:LsdException Locked="false" Priority="9" QFormat="true" Name="heading 7"/>
| + | |
| - | <w:LsdException Locked="false" Priority="9" QFormat="true" Name="heading 8"/>
| + | |
| - | <w:LsdException Locked="false" Priority="9" QFormat="true" Name="heading 9"/>
| + | |
| - | <w:LsdException Locked="false" Priority="39" Name="toc 1"/>
| + | |
| - | <w:LsdException Locked="false" Priority="39" Name="toc 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="39" Name="toc 3"/>
| + | |
| - | <w:LsdException Locked="false" Priority="39" Name="toc 4"/>
| + | |
| - | <w:LsdException Locked="false" Priority="39" Name="toc 5"/>
| + | |
| - | <w:LsdException Locked="false" Priority="39" Name="toc 6"/>
| + | |
| - | <w:LsdException Locked="false" Priority="39" Name="toc 7"/>
| + | |
| - | <w:LsdException Locked="false" Priority="39" Name="toc 8"/>
| + | |
| - | <w:LsdException Locked="false" Priority="39" Name="toc 9"/>
| + | |
| - | <w:LsdException Locked="false" Priority="35" QFormat="true" Name="caption"/>
| + | |
| - | <w:LsdException Locked="false" Priority="10" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" QFormat="true" Name="Title"/>
| + | |
| - | <w:LsdException Locked="false" Priority="1" Name="Default Paragraph Font"/>
| + | |
| - | <w:LsdException Locked="false" Priority="11" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" QFormat="true" Name="Subtitle"/>
| + | |
| - | <w:LsdException Locked="false" Priority="22" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" QFormat="true" Name="Strong"/>
| + | |
| - | <w:LsdException Locked="false" Priority="20" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" QFormat="true" Name="Emphasis"/>
| + | |
| - | <w:LsdException Locked="false" Priority="59" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Table Grid"/>
| + | |
| - | <w:LsdException Locked="false" UnhideWhenUsed="false" Name="Placeholder Text"/>
| + | |
| - | <w:LsdException Locked="false" Priority="1" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" QFormat="true" Name="No Spacing"/>
| + | |
| - | <w:LsdException Locked="false" Priority="60" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light Shading"/>
| + | |
| - | <w:LsdException Locked="false" Priority="61" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light List"/>
| + | |
| - | <w:LsdException Locked="false" Priority="62" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light Grid"/>
| + | |
| - | <w:LsdException Locked="false" Priority="63" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Shading 1"/>
| + | |
| - | <w:LsdException Locked="false" Priority="64" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Shading 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="65" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium List 1"/>
| + | |
| - | <w:LsdException Locked="false" Priority="66" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium List 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="67" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 1"/>
| + | |
| - | <w:LsdException Locked="false" Priority="68" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="69" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 3"/>
| + | |
| - | <w:LsdException Locked="false" Priority="70" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Dark List"/>
| + | |
| - | <w:LsdException Locked="false" Priority="71" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful Shading"/>
| + | |
| - | <w:LsdException Locked="false" Priority="72" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful List"/>
| + | |
| - | <w:LsdException Locked="false" Priority="73" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful Grid"/>
| + | |
| - | <w:LsdException Locked="false" Priority="60" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light Shading Accent 1"/>
| + | |
| - | <w:LsdException Locked="false" Priority="61" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light List Accent 1"/>
| + | |
| - | <w:LsdException Locked="false" Priority="62" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light Grid Accent 1"/>
| + | |
| - | <w:LsdException Locked="false" Priority="63" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Shading 1 Accent 1"/>
| + | |
| - | <w:LsdException Locked="false" Priority="64" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Shading 2 Accent 1"/>
| + | |
| - | <w:LsdException Locked="false" Priority="65" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium List 1 Accent 1"/>
| + | |
| - | <w:LsdException Locked="false" UnhideWhenUsed="false" Name="Revision"/>
| + | |
| - | <w:LsdException Locked="false" Priority="34" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" QFormat="true" Name="List Paragraph"/>
| + | |
| - | <w:LsdException Locked="false" Priority="29" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" QFormat="true" Name="Quote"/>
| + | |
| - | <w:LsdException Locked="false" Priority="30" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" QFormat="true" Name="Intense Quote"/>
| + | |
| - | <w:LsdException Locked="false" Priority="66" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium List 2 Accent 1"/>
| + | |
| - | <w:LsdException Locked="false" Priority="67" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 1 Accent 1"/>
| + | |
| - | <w:LsdException Locked="false" Priority="68" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 2 Accent 1"/>
| + | |
| - | <w:LsdException Locked="false" Priority="69" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 3 Accent 1"/>
| + | |
| - | <w:LsdException Locked="false" Priority="70" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Dark List Accent 1"/>
| + | |
| - | <w:LsdException Locked="false" Priority="71" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful Shading Accent 1"/>
| + | |
| - | <w:LsdException Locked="false" Priority="72" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful List Accent 1"/>
| + | |
| - | <w:LsdException Locked="false" Priority="73" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful Grid Accent 1"/>
| + | |
| - | <w:LsdException Locked="false" Priority="60" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light Shading Accent 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="61" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light List Accent 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="62" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light Grid Accent 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="63" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Shading 1 Accent 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="64" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Shading 2 Accent 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="65" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium List 1 Accent 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="66" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium List 2 Accent 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="67" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 1 Accent 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="68" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 2 Accent 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="69" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 3 Accent 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="70" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Dark List Accent 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="71" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful Shading Accent 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="72" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful List Accent 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="73" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful Grid Accent 2"/>
| + | |
| - | <w:LsdException Locked="false" Priority="60" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light Shading Accent 3"/>
| + | |
| - | <w:LsdException Locked="false" Priority="61" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light List Accent 3"/>
| + | |
| - | <w:LsdException Locked="false" Priority="62" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light Grid Accent 3"/>
| + | |
| - | <w:LsdException Locked="false" Priority="63" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Shading 1 Accent 3"/>
| + | |
| - | <w:LsdException Locked="false" Priority="64" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Shading 2 Accent 3"/>
| + | |
| - | <w:LsdException Locked="false" Priority="65" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium List 1 Accent 3"/>
| + | |
| - | <w:LsdException Locked="false" Priority="66" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium List 2 Accent 3"/>
| + | |
| - | <w:LsdException Locked="false" Priority="67" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 1 Accent 3"/>
| + | |
| - | <w:LsdException Locked="false" Priority="68" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 2 Accent 3"/>
| + | |
| - | <w:LsdException Locked="false" Priority="69" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 3 Accent 3"/>
| + | |
| - | <w:LsdException Locked="false" Priority="70" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Dark List Accent 3"/>
| + | |
| - | <w:LsdException Locked="false" Priority="71" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful Shading Accent 3"/>
| + | |
| - | <w:LsdException Locked="false" Priority="72" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful List Accent 3"/>
| + | |
| - | <w:LsdException Locked="false" Priority="73" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful Grid Accent 3"/>
| + | |
| - | <w:LsdException Locked="false" Priority="60" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light Shading Accent 4"/>
| + | |
| - | <w:LsdException Locked="false" Priority="61" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light List Accent 4"/>
| + | |
| - | <w:LsdException Locked="false" Priority="62" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light Grid Accent 4"/>
| + | |
| - | <w:LsdException Locked="false" Priority="63" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Shading 1 Accent 4"/>
| + | |
| - | <w:LsdException Locked="false" Priority="64" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Shading 2 Accent 4"/>
| + | |
| - | <w:LsdException Locked="false" Priority="65" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium List 1 Accent 4"/>
| + | |
| - | <w:LsdException Locked="false" Priority="66" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium List 2 Accent 4"/>
| + | |
| - | <w:LsdException Locked="false" Priority="67" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 1 Accent 4"/>
| + | |
| - | <w:LsdException Locked="false" Priority="68" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 2 Accent 4"/>
| + | |
| - | <w:LsdException Locked="false" Priority="69" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 3 Accent 4"/>
| + | |
| - | <w:LsdException Locked="false" Priority="70" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Dark List Accent 4"/>
| + | |
| - | <w:LsdException Locked="false" Priority="71" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful Shading Accent 4"/>
| + | |
| - | <w:LsdException Locked="false" Priority="72" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful List Accent 4"/>
| + | |
| - | <w:LsdException Locked="false" Priority="73" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful Grid Accent 4"/>
| + | |
| - | <w:LsdException Locked="false" Priority="60" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light Shading Accent 5"/>
| + | |
| - | <w:LsdException Locked="false" Priority="61" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light List Accent 5"/>
| + | |
| - | <w:LsdException Locked="false" Priority="62" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light Grid Accent 5"/>
| + | |
| - | <w:LsdException Locked="false" Priority="63" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Shading 1 Accent 5"/>
| + | |
| - | <w:LsdException Locked="false" Priority="64" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Shading 2 Accent 5"/>
| + | |
| - | <w:LsdException Locked="false" Priority="65" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium List 1 Accent 5"/>
| + | |
| - | <w:LsdException Locked="false" Priority="66" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium List 2 Accent 5"/>
| + | |
| - | <w:LsdException Locked="false" Priority="67" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 1 Accent 5"/>
| + | |
| - | <w:LsdException Locked="false" Priority="68" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 2 Accent 5"/>
| + | |
| - | <w:LsdException Locked="false" Priority="69" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 3 Accent 5"/>
| + | |
| - | <w:LsdException Locked="false" Priority="70" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Dark List Accent 5"/>
| + | |
| - | <w:LsdException Locked="false" Priority="71" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful Shading Accent 5"/>
| + | |
| - | <w:LsdException Locked="false" Priority="72" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful List Accent 5"/>
| + | |
| - | <w:LsdException Locked="false" Priority="73" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful Grid Accent 5"/>
| + | |
| - | <w:LsdException Locked="false" Priority="60" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light Shading Accent 6"/>
| + | |
| - | <w:LsdException Locked="false" Priority="61" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light List Accent 6"/>
| + | |
| - | <w:LsdException Locked="false" Priority="62" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Light Grid Accent 6"/>
| + | |
| - | <w:LsdException Locked="false" Priority="63" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Shading 1 Accent 6"/>
| + | |
| - | <w:LsdException Locked="false" Priority="64" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Shading 2 Accent 6"/>
| + | |
| - | <w:LsdException Locked="false" Priority="65" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium List 1 Accent 6"/>
| + | |
| - | <w:LsdException Locked="false" Priority="66" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium List 2 Accent 6"/>
| + | |
| - | <w:LsdException Locked="false" Priority="67" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 1 Accent 6"/>
| + | |
| - | <w:LsdException Locked="false" Priority="68" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 2 Accent 6"/>
| + | |
| - | <w:LsdException Locked="false" Priority="69" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Medium Grid 3 Accent 6"/>
| + | |
| - | <w:LsdException Locked="false" Priority="70" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Dark List Accent 6"/>
| + | |
| - | <w:LsdException Locked="false" Priority="71" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful Shading Accent 6"/>
| + | |
| - | <w:LsdException Locked="false" Priority="72" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful List Accent 6"/>
| + | |
| - | <w:LsdException Locked="false" Priority="73" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" Name="Colorful Grid Accent 6"/>
| + | |
| - | <w:LsdException Locked="false" Priority="19" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" QFormat="true" Name="Subtle Emphasis"/>
| + | |
| - | <w:LsdException Locked="false" Priority="21" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" QFormat="true" Name="Intense Emphasis"/>
| + | |
| - | <w:LsdException Locked="false" Priority="31" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" QFormat="true" Name="Subtle Reference"/>
| + | |
| - | <w:LsdException Locked="false" Priority="32" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" QFormat="true" Name="Intense Reference"/>
| + | |
| - | <w:LsdException Locked="false" Priority="33" SemiHidden="false"
| + | |
| - | UnhideWhenUsed="false" QFormat="true" Name="Book Title"/>
| + | |
| - | <w:LsdException Locked="false" Priority="37" Name="Bibliography"/>
| + | |
| - | <w:LsdException Locked="false" Priority="39" QFormat="true" Name="TOC Heading"/>
| + | |
| - | </w:LatentStyles>
| + | |
| - | </xml><![endif]-->
| + | |
| - | <style>
| + | |
| - | <!--
| + | |
| - | /* Font Definitions */
| + | |
| - | @font-face
| + | |
| - | {font-family:Calibri;
| + | |
| - | panose-1:2 15 5 2 2 2 4 3 2 4;
| + | |
| - | mso-font-charset:0;
| + | |
| - | mso-generic-font-family:swiss;
| + | |
| - | mso-font-pitch:variable;
| + | |
| - | mso-font-signature:-520092929 1073786111 9 0 415 0;}
| + | |
| - | /* Style Definitions */
| + | |
| - | p.MsoNormal, li.MsoNormal, div.MsoNormal
| + | |
| - | {mso-style-unhide:no;
| + | |
| - | mso-style-qformat:yes;
| + | |
| - | mso-style-parent:"";
| + | |
| - | margin:0in;
| + | |
| - | margin-bottom:.0001pt;
| + | |
| - | mso-pagination:widow-orphan;
| + | |
| - | font-size:11.0pt;
| + | |
| - | font-family:"Calibri","sans-serif";
| + | |
| - | mso-ascii-font-family:Calibri;
| + | |
| - | mso-ascii-theme-font:minor-latin;
| + | |
| - | mso-fareast-font-family:Calibri;
| + | |
| - | mso-fareast-theme-font:minor-latin;
| + | |
| - | mso-hansi-font-family:Calibri;
| + | |
| - | mso-hansi-theme-font:minor-latin;
| + | |
| - | mso-bidi-font-family:"Times New Roman";
| + | |
| - | mso-bidi-theme-font:minor-bidi;}
| + | |
| - | span.SpellE
| + | |
| - | {mso-style-name:"";
| + | |
| - | mso-spl-e:yes;}
| + | |
| - | span.GramE
| + | |
| - | {mso-style-name:"";
| + | |
| - | mso-gram-e:yes;}
| + | |
| - | .MsoChpDefault
| + | |
| - | {mso-style-type:export-only;
| + | |
| - | mso-default-props:yes;
| + | |
| - | font-family:"Calibri","sans-serif";
| + | |
| - | mso-ascii-font-family:Calibri;
| + | |
| - | mso-ascii-theme-font:minor-latin;
| + | |
| - | mso-fareast-font-family:Calibri;
| + | |
| - | mso-fareast-theme-font:minor-latin;
| + | |
| - | mso-hansi-font-family:Calibri;
| + | |
| - | mso-hansi-theme-font:minor-latin;
| + | |
| - | mso-bidi-font-family:"Times New Roman";
| + | |
| - | mso-bidi-theme-font:minor-bidi;}
| + | |
| - | @page WordSection1
| + | |
| - | {size:8.5in 11.0in;
| + | |
| - | margin:1.0in 1.0in 1.0in 1.0in;
| + | |
| - | mso-header-margin:.5in;
| + | |
| - | mso-footer-margin:.5in;
| + | |
| - | mso-paper-source:0;}
| + | |
| - | div.WordSection1
| + | |
| - | {page:WordSection1;}
| + | |
| - | -->
| + | |
| - | </style> | + | |
| - | <!--[if gte mso 10]> | + | |
| - | <style>
| + | |
| - | /* Style Definitions */
| + | |
| - | table.MsoNormalTable
| + | |
| - | {mso-style-name:"Table Normal";
| + | |
| - | mso-tstyle-rowband-size:0;
| + | |
| - | mso-tstyle-colband-size:0;
| + | |
| - | mso-style-noshow:yes;
| + | |
| - | mso-style-priority:99;
| + | |
| - | mso-style-parent:"";
| + | |
| - | mso-padding-alt:0in 5.4pt 0in 5.4pt;
| + | |
| - | mso-para-margin:0in;
| + | |
| - | mso-para-margin-bottom:.0001pt;
| + | |
| - | mso-pagination:widow-orphan;
| + | |
| - | font-size:11.0pt;
| + | |
| - | font-family:"Calibri","sans-serif";
| + | |
| - | mso-ascii-font-family:Calibri;
| + | |
| - | mso-ascii-theme-font:minor-latin;
| + | |
| - | mso-hansi-font-family:Calibri;
| + | |
| - | mso-hansi-theme-font:minor-latin;
| + | |
| - | mso-bidi-font-family:"Times New Roman";
| + | |
| - | mso-bidi-theme-font:minor-bidi;}
| + | |
| - | </style>
| + | |
| - | <![endif]--><!--[if gte mso 9]><xml>
| + | |
| - | <o:shapedefaults v:ext="edit" spidmax="1026"/>
| + | |
| - | </xml><![endif]--><!--[if gte mso 9]><xml>
| + | |
| - | <o:shapelayout v:ext="edit">
| + | |
| - | <o:idmap v:ext="edit" data="1"/>
| + | |
| - | </o:shapelayout></xml><![endif]-->
| + | |
| - | </head>
| + | |
| | | | |
| - | <body lang=EN-US style='tab-interval:.5in'>
| |
| | | | |
| - | <div class=WordSection1>
| + | ==Experiment 2: Construction of a refactored decaffeination operon== |
| | | | |
| - | <p class=MsoNormal><p><o:p></o:p></p> | + | 2a. Phusion PCR<br/> |
| | | | |
| - | <p class=MsoNormal>Experiment 2: <span class=SpellE>Constructon</span> of a | + | Primers:<br/> |
| - | refactored decaffeination operon<o:p></o:p></p>
| + | EQ_pSB1c3_NdmA_for: TAGGTACAGTGCTAGCTACTAGAGAAATCAAATTAAGGAGGTAAGATAAATGGAACAGGCAATCATTAATGATGAACGGG<br/> |
| | + | EQ_pBSC1C_NdmA_2: TGATTTCTGGAATTCGCGGCCGCTTCTAGAGATTAAGGAGGTAAGATAAATGGAACAGGCAATCATTAATGATGAACGGG<br/> |
| | + | EQ_pSB1C3_Gib_rev: TTGATTTCTCTAGTAGCTAGCACTGTACCTAGGACTG<br/> |
| | + | EQ_NdmA_rev: TTATATGTAGCTCCTATCGCTTTCAATGACTGGG<br/> |
| | + | EQ_RBS_NdmB_for: gtCATTGAAAGCGATAGGAGCTACATATAATCTAGAGAAAGAGGAGAAATACTAGATGAAAGAACAGCTCAAGCCGCTG<br/> |
| | + | EQ_NdmB_rev: ccggtctcgcTTACTGTTCTTCTTCAATAACATTCGTCAAGACG<br/> |
| | + | EQ_RBS_NdmC_for: GAAGAAGAACAGTAAgcgagaccggTCTAGAGAAAGAGGAGAAATACTAGATGTCTACTGACCAAGTAATTTTTAACGactgg<br/> |
| | + | EQ_NdmC_rev: TTAGTCCCGCAGAGCACCATATTGCac<br/> |
| | + | EQ_RBS_NdmD for: GCAATATGGTGCTCTGCGGGACTAATCTAGAGAAAGAGGAGAAATACTAGATGAACAAACTTGACGTCAACCagtgg<br/> |
| | + | EQ_NdmD_pBS1C_rev: TTATACAGCTCGTCCATACCGTGGGTGATGCCCGGCCTCACAGATCGAGAACGATT<br/> |
| | + | EQ_pBS1C_Gib_for: GGGCATCACCCACGGTATGGACGAG<br/> |
| | + | EQ_pBS1C_rev_2: CTCTAGAAGCGGCCGCGAATTCCAGAAATCA<br/> |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| |
| | | | |
| - | <p class=MsoNormal>2a<span class=GramE>.<span style='mso-spacerun:yes'>
| |
| - | </span><span class=SpellE>Phusion</span></span> PCR<o:p></o:p></p>
| |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p> | + | 10 uL 5x Phusion HF Buffer<br/> |
| | + | 1 uL 10mM dNTPs<br/> |
| | + | 5 uL 5uM forward primer<br/> |
| | + | 5 uL 5uM reverse primer<br/> |
| | + | 1 uL DMSO<br/> |
| | + | 1 uL template<br/> |
| | + | 26.5 uL H20<br/> |
| | + | --<br/> |
| | + | .5 uL phusion polymerase<br/> |
| | | | |
| - | <p class=MsoNormal>Primers:<o:p></o:p></p>
| + | rxns:<br/> |
| | | | |
| - | <p class=MsoNormal>EQ_pSB1c3_NdmA_for: | + | Vector PCRs<br/> |
| - | TAGGTACAGTGCTAGCTACTAGAGAAATCAAATTAAGGAGGTAAGATAAATGGAACAGGCAATCATTAATGATGAACGGG<o:p></o:p></p>
| + | 1. Bba_k515105 + EQ_pSB1C3_Gib_for + EQ_pSB1c3_gib_rev<br/> |
| | + | 2. Bba_k515105 + EQ_pSB1C3_Gib_for + EQ_pSB1c3_gib_rev_2<br/> |
| | | | |
| - | <p class=MsoNormal>EQ_pBSC1C_NdmA_2: | + | Insert PCRs<br/> |
| - | TGATTTCTGGAATTCGCGGCCGCTTCTAGAGATTAAGGAGGTAAGATAAATGGAACAGGCAATCATTAATGATGAACGGG<o:p></o:p></p>
| + | 3. CBB5 cells + EQ_NdmA_for + EQ_NdmA_rev<br/> |
| | + | 4. CBB5 cells + EQ_pSB1C3_NdmA_2 + EQ_NdmA_rev<br/> |
| | + | 5. CBB5 cells + EQ_RBS_NdmB_for + EQ_NdmB_rev<br/> |
| | + | 6. CBB5 cells + EQ_RBS_NdmC_for + EQ_NdmC_rev<br/> |
| | + | 7. CBB5 cells + EQ_RBS_NdmD_for + EQ_NdmD_pSB1C_rev<br/> |
| | | | |
| - | <p class=MsoNormal>EQ_pSB1C3_Gib_rev: TTGATTTCTCTAGTAGCTAGCACTGTACCTAGGACTG<o:p></o:p></p>
| + | PCR cycling:<br/> |
| | | | |
| - | <p class=MsoNormal><span class=SpellE>EQ_NdmA_rev</span>: | + | 98 deg x 3 min<br/> |
| - | TTATATGTAGCTCCTATCGCTTTCAATGACTGGG<o:p></o:p></p>
| + | --<br/> |
| | + | 98 deg x 30s<br/> |
| | + | 58 deg x 20s x30 cycles<br/> |
| | + | 72 deg x 2 min<br/> |
| | + | --<br/> |
| | + | 72 deg x 10 min<br/> |
| | | | |
| - | <p class=MsoNormal><span class=SpellE>EQ_RBS_NdmB_for</span>:
| |
| - | gtCATTGAAAGCGATAGGAGCTACATATAATCTAGAGAAAGAGGAGAAATACTAGATGAAAGAACAGCTCAAGCCGCTG<o:p></o:p></p>
| |
| | | | |
| - | <p class=MsoNormal><span class=SpellE>EQ_NdmB_rev</span>: <span class=SpellE>ccggtctcgcTTACTGTTCTTCTTCAATAACATTCGTCAAGACG</span><o:p></o:p></p>
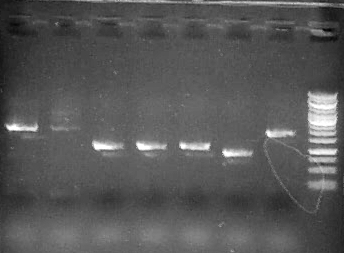
| + | Run 1 uL 2a1-7, 3.5 uL Fermentas 1kb GeneRuler ladder |
| | | | |
| - | <p class=MsoNormal><span class=SpellE>EQ_RBS_NdmC_for</span>:
| + | [[File:2a.jpg]] |
| - | GAAGAAGAACAGTAAgcgagaccggTCTAGAGAAAGAGGAGAAATACTAGATGTCTACTGACCAAGTAATTTTTAACGactgg<o:p></o:p></p>
| + | |
| | | | |
| - | <p class=MsoNormal><span class=SpellE>EQ_NdmC_rev</span>: <span class=SpellE>TTAGTCCCGCAGAGCACCATATTGCac</span><o:p></o:p></p>
| |
| | | | |
| - | <p class=MsoNormal><span class=SpellE>EQ_RBS_NdmD</span> for:
| |
| - | GCAATATGGTGCTCTGCGGGACTAATCTAGAGAAAGAGGAGAAATACTAGATGAACAAACTTGACGTCAACCagtgg<o:p></o:p></p>
| |
| | | | |
| - | <p class=MsoNormal>EQ_NdmD_pBS1C_rev: | + | 2b. PCR purification<br/> |
| - | TTATACAGCTCGTCCATACCGTGGGTGATGCCCGGCCTCACAGATCGAGAACGATT<o:p></o:p></p>
| + | |
| | | | |
| - | <p class=MsoNormal>EQ_pBS1C_Gib_for: GGGCATCACCCACGGTATGGACGAG<o:p></o:p></p> | + | Qiagen PCR purification protocol<br/> |
| | | | |
| - | <p class=MsoNormal>EQ_pBS1C_rev_2: CTCTAGAAGCGGCCGCGAATTCCAGAAATCA<o:p></o:p></p> | + | Elute in 35 uL h20<br/> |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | nanodrop concentrations:<br/> |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p> | + | 1. vector 1: 289.0 ng/uL<br/> |
| | + | 2. vector 2: 85.6 ng/uL<br/> |
| | + | 3. NdmA_1: 139.8 ng/uL<br/> |
| | + | 4. NdmA_2: 144.5 ngl/uL<br/> |
| | + | 5. NdmB: 144.6ng/uL<br/> |
| | + | 6. NdmC: 146.1 ng/uL<br/> |
| | + | 7. NdmD: 104.8ng/uL<br/> |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | 2c: DpnI digest of vector PCRs<br/> |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p> | + | 1.<br/> |
| | + | 15 uL vector 1<br/> |
| | + | 5 uL 10x NEB 4 Buffer<br/> |
| | + | 1 uL dpnI<br/> |
| | + | 29 uL H20<br/> |
| | + | --<br/> |
| | + | 50 uL <br/> |
| | | | |
| - | <p class=MsoNormal>10 <span class=SpellE>uL</span> 5x <span class=SpellE>Phusion</span> | + | 2.<br/> |
| - | HF Buffer<o:p></o:p></p>
| + | 30 uL vector 2<br/> |
| | + | 5 uL 10x NEB 4 Buffer<br/> |
| | + | 1 uL dpnI<br/> |
| | + | 14 uL H20<br/> |
| | + | --<br/> |
| | + | 50 uL<br/> |
| | | | |
| - | <p class=MsoNormal>1 <span class=SpellE>uL</span> 10mM <span class=SpellE>dNTPs</span><o:p></o:p></p> | + | 37 deg x 2 hrs<br/> |
| | | | |
| - | <p class=MsoNormal>5 <span class=SpellE>uL</span> 5uM forward primer<o:p></o:p></p> | + | 2e. Purification<br/> |
| | | | |
| - | <p class=MsoNormal>5 <span class=SpellE>uL</span> 5uM reverse primer<o:p></o:p></p> | + | Standard Qiagen PCR purification protocol. Elute in H20.<br/> |
| | | | |
| - | <p class=MsoNormal>1 <span class=SpellE>uL</span> DMSO<o:p></o:p></p>
| |
| | | | |
| - | <p class=MsoNormal>1 <span class=SpellE>uL</span> template<o:p></o:p></p>
| |
| | | | |
| - | <p class=MsoNormal>26.5 <span class=SpellE>uL</span> H20<o:p></o:p></p>
| + | 2F. Gibson cloning<br/> |
| | | | |
| - | <p class=MsoNormal>--<o:p></o:p></p>
| + | pmols = weight(ng) x 1000 / (bp x 650 daltons)<br/> |
| | | | |
| - | <p class=MsoNormal>.5 <span class=SpellE>uL</span> <span class=SpellE>phusion</span>
| |
| - | polymerase<o:p></o:p></p>
| |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p> | + | Will use .05 pmol of vector, .1 pmol for each insert<br/> |
| | | | |
| - | <p class=MsoNormal><span class=SpellE><span class=GramE>rxns</span></span>:<o:p></o:p></p>
| + | Vec 1 (.05pmol) = need 68.57ng / (79.5ng/uL) = .86uL<br/> |
| | + | Vec 2 (.05pmol) = need 68.57ng / (132.5ng/uL) = .52uL<br/> |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p> | + | NdmA1 (.1pmol) = need 71.5ng / (139.8ng/uL) = .51 uL<br/> |
| | + | NdmA2 (.1pmol) = need 71.5ng / (144.5ng/uL) = .49 uL<br/> |
| | + | NdmB (.1pmol) = need 73.4ng / (144.6ng/uL) = .50 uL<br/> |
| | + | NdmC (.1pmol) = need 58.8ng / (146.1ng/uL) = .40 uL<br/> |
| | + | NdmD (.1pmol) = need 120.ng / (104.8ng/uL) = 1.15 uL<br/> |
| | | | |
| - | <p class=MsoNormal>Vector PCRs<o:p></o:p></p>
| + | rxns:<br/> |
| | | | |
| - | <p class=MsoNormal>1. Bba_<span class=GramE>k515105<span | + | 1.<br/> |
| - | style='mso-spacerun:yes'> </span>+</span> EQ_pSB1C3_Gib_for +
| + | .86 uL vec 1<br/> |
| - | EQ_pSB1c3_gib_rev<o:p></o:p></p>
| + | .51 uL ndmA_1<br/> |
| | + | .50 uL NdmB<br/> |
| | + | .40 uL NdmC<br/> |
| | + | 1.15 uL NdmD<br/> |
| | + | 15 ul 1.33x Gibson Master Mix<br/> |
| | + | 1.58 uL H20<br/> |
| | + | --<br/> |
| | + | 20 uL total<br/> |
| | | | |
| - | <p class=MsoNormal>2. Bba_<span class=GramE>k515105<span
| + | 2.<br/> |
| - | style='mso-spacerun:yes'> </span>+</span> EQ_pSB1C3_Gib_for +
| + | .86 uL vec 1<br/> |
| - | EQ_pSB1c3_gib_rev_2<o:p></o:p></p>
| + | 15 uL 1.33x Gibson Master Mix<br/> |
| | + | 4.14 uL H20<br/> |
| | + | --<br/> |
| | + | 20 uL total<br/> |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p> | + | 3.<br/> |
| | + | .52 uL Vec 2<br/> |
| | + | .51 uL ndmA_1<br/> |
| | + | .50 uL NdmB<br/> |
| | + | .40 uL NdmC<br/> |
| | + | 1.15 uL NdmD<br/> |
| | + | 15 ul 1.33x Gibson Master Mix<br/> |
| | + | 1.94 uL H20<br/> |
| | + | --<br/> |
| | + | 20 uL total<br/> |
| | | | |
| - | <p class=MsoNormal>Insert PCRs<o:p></o:p></p> | + | 4.<br/> |
| | + | .52 uL vec 1<br/> |
| | + | 15 uL 1.33x Gibson Master Mix<br/> |
| | + | 4.14 uL H20<br/> |
| | + | --<br/> |
| | + | 20 uL total<br/> |
| | | | |
| - | <p class=MsoNormal>3. CBB5 cells + <span class=SpellE>EQ_NdmA_for</span> + <span | + | 50 deg thermocycler x 60 minutes<br/> |
| - | class=SpellE>EQ_NdmA_rev</span><o:p></o:p></p>
| + | |
| | | | |
| - | <p class=MsoNormal>4. CBB5 cells + EQ_pSB1C3_NdmA_2 + <span class=SpellE>EQ_NdmA_rev</span><o:p></o:p></p>
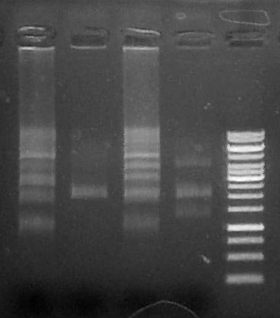
| + | Run 5 uL each reaction (1-4)on .8% agarose gel + 3.5 ul Fermentas 1kb GeneRuler<br/> |
| | | | |
| - | <p class=MsoNormal>5. CBB5 cells + <span class=SpellE>EQ_RBS_NdmB_for</span> + <span
| + | [[File:2f.jpg]] |
| - | class=SpellE>EQ_NdmB_rev</span><o:p></o:p></p>
| + | |
| | | | |
| - | <p class=MsoNormal>6. CBB5 cells + <span class=SpellE>EQ_RBS_NdmC_for</span> + <span
| |
| - | class=SpellE>EQ_NdmC_rev</span><o:p></o:p></p>
| |
| | | | |
| - | <p class=MsoNormal>7. CBB5 cells + <span class=SpellE>EQ_RBS_NdmD_for</span> +
| + | Desalted gibson reactions on .025uM Nitrocellulose membranes x 20 minutes<br/> |
| - | EQ_NdmD_pSB1C_rev<o:p></o:p></p>
| + | |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p> | + | 2g. Electroporation<br/> |
| | | | |
| - | <p class=MsoNormal>PCR cycling:<o:p></o:p></p> | + | Decided to not proceed with promoterless construct (2F3,4)<br/> |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | 1 uL desalted gibson reaction (2F1,2) ->50 uL electrocompetent BW25113-GuaB -> 1mL SOC -> 1hr incubation @ 37 deg<br/> |
| | | | |
| - | <p class=MsoNormal>98 <span class=SpellE>deg</span> x 3 min<o:p></o:p></p>
| |
| | | | |
| - | <p class=MsoNormal>--<o:p></o:p></p>
| + | 2j. Selective growth in Caffeine/Theophylline<br/> |
| | | | |
| - | <p class=MsoNormal>98 <span class=SpellE>deg</span> x 30s<o:p></o:p></p>
| + | dilute 60 uL 2g transformations (1-2) -> 3mL Mineral M9 media + 34ug/mL chloramphenicol +/- 100mg Caffeine or Theophylline. Place on 30 deg shaker x 48 hrs<br/> |
| | | | |
| - | <p class=MsoNormal>58 <span class=SpellE>deg</span> x 20s<span
| + | Results (growth): |
| - | style='mso-spacerun:yes'> </span>x30 cycles<o:p></o:p></p>
| + | {| cellpadding="5" border="1" cellspacing="0" |
| | + | ! Media |
| | + | ! 2g1 (+operon) |
| | + | ! 2g2 (vector only) |
| | + | |- |
| | + | | M9 |
| | + | | - |
| | + | | - |
| | + | |- |
| | + | | M9 + Caffeine |
| | + | | - |
| | + | | - |
| | + | |- |
| | + | | M9 + Theophylline |
| | + | | + |
| | + | | - |
| | + | |- |
| | + | |} |
| | | | |
| - | <p class=MsoNormal>72 <span class=SpellE>deg</span> x 2 min<o:p></o:p></p>
| |
| | | | |
| - | <p class=MsoNormal>--<o:p></o:p></p>
| |
| | | | |
| - | <p class=MsoNormal>72 <span class=SpellE>deg</span> x 10 min<o:p></o:p></p>
| + | Result: construct enables growth (demethylation) on Theophylline. NdmC is not fuctional.<br/> |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p> | + | Streak 2j1 theophylline enriched culture onto LB-chloramphenicol plate for isolation of single colonies.<br/> |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p> | + | 2K Plasmid Prep<br/> |
| | | | |
| - | <p class=MsoNormal>Gel igm2a<o:p></o:p></p> | + | Pick 2 colonies from theophylline enriched streak for plasmid prep. Prep 5mL culture.<br/> |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p> | + | 2L Restriction Digest<br/> |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p> | + | Perform restriction digest to analyze insert size of plasmids<br/> |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p> | + | 1 uL 2k1,2<br/> |
| | + | 1 uL 10x NEB 3<br/> |
| | + | .5 uL NotI<br/> |
| | + | 6.5 uL H20<br/> |
| | + | --<br/> |
| | + | 10 uL<br/> |
| | | | |
| - | <p class=MsoNormal>2b. PCR purification<o:p></o:p></p> | + | 37 deg x 1hr<br/> |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p> | + | Run 5 uL on .8% agarose gel<br/> |
| | | | |
| - | <p class=MsoNormal><span class=SpellE>Qiagen</span> PCR purification protocol<o:p></o:p></p>
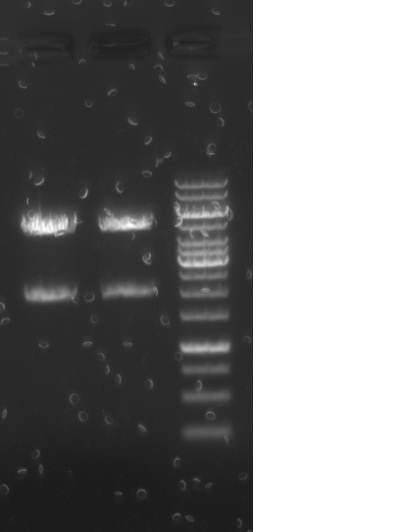
| + | [[File:2L.jpg|200px]]<br/> |
| | | | |
| - | <p class=MsoNormal><o:p> </o:p></p> | + | Lanes: <br/> |
| | + | 1. 2k1<br/> |
| | + | 2. 2k2<br/> |
| | + | 3. GeneRuler 1kb ladder |
| | | | |
| - | <p class=MsoNormal>Elute in 35 <span class=SpellE>uL</span> h20<o:p></o:p></p>
| + | Result: both clones show anticipated ~5kB band corresponding to the complete operon.<br/> |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><span class=SpellE><span class=GramE>nanodrop</span></span>
| + | |
| - | concentrations:<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>1. <span class=GramE>vector</span> 1: 289.0 <span
| + | |
| - | class=SpellE>ng</span>/<span class=SpellE>uL</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>2. <span class=GramE>vector</span> 2: 85.6 <span
| + | |
| - | class=SpellE>ng</span>/<span class=SpellE>uL</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>3. NdmA_1: <span class=GramE>139.8 <span class=SpellE>ng</span>/<span
| + | |
| - | class=SpellE>uL</span></span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>4. NdmA_2: <span class=GramE>144.5 <span class=SpellE>ngl</span>/<span
| + | |
| - | class=SpellE>uL</span></span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>5. <span class=SpellE>NdmB</span>: 144.6ng/<span
| + | |
| - | class=SpellE>uL</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>6. <span class=SpellE>NdmC</span>: <span class=GramE>146.1 <span
| + | |
| - | class=SpellE>ng</span>/<span class=SpellE>uL</span></span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>7. <span class=SpellE>NdmD</span>: 104.8ng/<span
| + | |
| - | class=SpellE>uL</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>2c: <span class=SpellE>DpnI</span> digest of vector PCRs<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>1.<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>15 <span class=SpellE>uL</span> vector 1<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>5 <span class=SpellE>uL</span> 10x NEB 4 Buffer<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>1 <span class=SpellE>uL</span> <span class=SpellE>dpnI</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>29 <span class=SpellE>uL</span> H20<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>--<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>50 <span class=SpellE>uL</span> <o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>2.<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>30 <span class=SpellE>uL</span> vector 2<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>5 <span class=SpellE>uL</span> 10x NEB 4 Buffer<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>1 <span class=SpellE>uL</span> <span class=SpellE>dpnI</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>14 <span class=SpellE>uL</span> H20<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>--<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>50 <span class=SpellE>uL</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>37 <span class=SpellE>deg</span> x 2 <span class=SpellE>hrs</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>2e. Purification<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><span class=GramE>Standard <span class=SpellE>Qiagen</span>
| + | |
| - | PCR purification protocol.</span><span style='mso-spacerun:yes'> </span>Elute
| + | |
| - | in H20.<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>2F. Gibson cloning<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><span class=SpellE><span class=GramE>pmols</span></span> =
| + | |
| - | weight(<span class=SpellE>ng</span>) x 1000 / (<span class=SpellE>bp</span> x
| + | |
| - | 650 <span class=SpellE>daltons</span>)<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>Will use .05 <span class=SpellE>pmol</span> of vector, .1 <span
| + | |
| - | class=SpellE>pmol</span> for each insert<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><span class=SpellE>Vec</span> 1 (.05pmol) = need 68.57ng /
| + | |
| - | (79.5ng/<span class=SpellE>uL</span>) = .86uL<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><span class=SpellE>Vec</span> 2 (.05pmol) = need 68.57ng /
| + | |
| - | (132.5ng/<span class=SpellE>uL</span>) = .52uL<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>NdmA1 (.1pmol) = need 71.5ng / (139.8ng/<span class=SpellE>uL</span>)
| + | |
| - | = .51 <span class=SpellE>uL</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>NdmA2 (.1pmol) = need 71.5ng / (144.5ng/<span class=SpellE>uL</span>)
| + | |
| - | = .49 <span class=SpellE>uL</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><span class=SpellE><span class=GramE>NdmB</span></span><span
| + | |
| - | class=GramE><span style='mso-spacerun:yes'> </span>(</span>.1pmol) = need
| + | |
| - | 73.4ng / (144.6ng/<span class=SpellE>uL</span>) = .50 <span class=SpellE>uL</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><span class=SpellE><span class=GramE>NdmC</span></span><span
| + | |
| - | class=GramE><span style='mso-spacerun:yes'> </span>(</span>.1pmol) = need
| + | |
| - | 58.8ng / (146.1ng/<span class=SpellE>uL</span>) = .40 <span class=SpellE>uL</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><span class=SpellE><span class=GramE>NdmD</span></span><span
| + | |
| - | class=GramE><span style='mso-spacerun:yes'> </span>(</span>.1pmol) = need
| + | |
| - | 120.ng / (104.8ng/<span class=SpellE>uL</span>) = 1.15 <span class=SpellE>uL</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><span class=SpellE><span class=GramE>rxns</span></span>:<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>1.<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>.86 <span class=SpellE>uL</span> <span class=SpellE>vec</span>
| + | |
| - | 1<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>.51 <span class=SpellE>uL</span> ndmA_1<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>.50 <span class=SpellE>uL</span> <span class=SpellE>NdmB</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>.40 <span class=SpellE>uL</span> <span class=SpellE>NdmC</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>1.15 <span class=SpellE>uL</span> <span class=SpellE>NdmD</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>15 <span class=SpellE>ul</span> 1.33x Gibson Master Mix<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>1.58 <span class=SpellE>uL</span> H20<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>--<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>20 <span class=SpellE>uL</span> total<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>2.<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>.86 <span class=SpellE>uL</span> <span class=SpellE>vec</span>
| + | |
| - | 1<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>15 <span class=SpellE>uL</span> 1.33x Gibson Master Mix<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>4.14 <span class=SpellE>uL</span> H20<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>--<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>20 <span class=SpellE>uL</span> total<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>3.<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>.52 <span class=SpellE>uL</span> <span class=SpellE>Vec</span>
| + | |
| - | 2<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>.51 <span class=SpellE>uL</span> ndmA_1<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>.50 <span class=SpellE>uL</span> <span class=SpellE>NdmB</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>.40 <span class=SpellE>uL</span> <span class=SpellE>NdmC</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>1.15 <span class=SpellE>uL</span> <span class=SpellE>NdmD</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>15 <span class=SpellE>ul</span> 1.33x Gibson Master Mix<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>1.94 <span class=SpellE>uL</span> H20<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>--<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>20 <span class=SpellE>uL</span> total<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>4.<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>.52 <span class=SpellE>uL</span> <span class=SpellE>vec</span>
| + | |
| - | 1<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>15 <span class=SpellE>uL</span> 1.33x Gibson Master Mix<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>4.14 <span class=SpellE>uL</span> H20<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>--<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>20 <span class=SpellE>uL</span> total<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>50 <span class=SpellE>deg</span> <span class=SpellE>thermocycler</span>
| + | |
| - | x 60 minutes<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>Run 5 <span class=SpellE>uL</span> each reaction on .8% <span
| + | |
| - | class=SpellE>agarose</span> gel<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><span class=GramE>insert</span> igm2f.jpeg<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>Desalted <span class=SpellE><span class=GramE>gibson</span></span>
| + | |
| - | reactions on .025uM Nitrocellulose membranes x 20 minutes<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>2g<span class=GramE>.<span style='mso-spacerun:yes'>
| + | |
| - | </span>Electroporation</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>Decided to not proceed with <span class=SpellE>promoterless</span>
| + | |
| - | construct (2F3<span class=GramE>,4</span>)<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>1 <span class=SpellE>uL</span> desalted <span class=SpellE>gibson</span>
| + | |
| - | reaction (2F1<span class=GramE>,2</span>) ->50 <span class=SpellE>uL</span> <span
| + | |
| - | class=SpellE>electrocompetent</span> BW25113-GuaB -> 1mL SOC -> 1hr
| + | |
| - | incubation @ 37 <span class=SpellE>deg</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>2j<span class=GramE>.<span style='mso-spacerun:yes'>
| + | |
| - | </span>Selective</span> growth in Caffeine/Theophylline<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>dilute 60 <span class=SpellE>uL</span> 2g transformations
| + | |
| - | (1-2) -> 3mL Mineral M9 media + 34ug/mL chloramphenicol +/- 100mg Caffeine
| + | |
| - | or Theophylline.<span style='mso-spacerun:yes'> </span>Place on 30 <span
| + | |
| - | class=SpellE>deg</span> shaker x 48 <span class=SpellE>hrs</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>Results (growth):<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><span style='mso-tab-count:3'> </span>2g1
| + | |
| - | (+operon)<span style='mso-tab-count:2'> </span>2g2 (vector
| + | |
| - | only)<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>1. M9<span style='mso-tab-count:3'> </span>-<span
| + | |
| - | style='mso-tab-count:3'> </span>-<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>2. M9 + caffeine<span style='mso-tab-count:1'> </span>-<span
| + | |
| - | style='mso-tab-count:3'> </span>-<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>3. M9 + Theophylline<span style='mso-tab-count:1'> </span>+<span
| + | |
| - | style='mso-tab-count:3'> </span>_<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>Result: construct enables growth (<span class=SpellE>demethylation</span>)
| + | |
| - | on Theophylline.<span style='mso-spacerun:yes'> </span><span class=SpellE>NdmC</span>
| + | |
| - | is not <span class=SpellE>fuctional</span>.<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>Streak 2j1 theophylline enriched culture onto
| + | |
| - | LB-chloramphenicol plate for isolation of single colonies.<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>2K Plasmid Prep<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>Pick 2 colonies from theophylline enriched streak for
| + | |
| - | plasmid prep.<span style='mso-spacerun:yes'> </span>Prep 5mL culture.<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>2L Restriction Digest<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>Perform restriction digest to analyze insert size of
| + | |
| - | plasmids<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>1 <span class=SpellE>uL</span> 2k1<span class=GramE>,2</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>1 <span class=SpellE>uL</span> 10x NEB 3<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>.5 <span class=SpellE>uL</span> <span class=SpellE>NotI</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>6.5 <span class=SpellE>uL</span> H20<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>--<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>10 <span class=SpellE>uL</span><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>37 <span class=SpellE>deg</span> x 1hr<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>Run 5 <span class=SpellE>uL</span> on .8% <span
| + | |
| - | class=SpellE>agarose</span> gel<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><span class=GramE>insert</span> gel image (on darkroom
| + | |
| - | computer?)<o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal>Result:<span style='mso-spacerun:yes'> </span>both clones
| + | |
| - | show anticipated ~5kB band corresponding to the complete operon.<o:p></o:p></p> | + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal></p><o:p></o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | <p class=MsoNormal><o:p> </o:p></p>
| + | |
| - | | + | |
| - | </div>
| + | |
| - | | + | |
| - | </body>
| + | |
| - | | + | |
| - | </html>
| + | |
2a. Phusion PCR
2b. PCR purification
1. vector 1: 289.0 ng/uL
2. vector 2: 85.6 ng/uL
3. NdmA_1: 139.8 ng/uL
4. NdmA_2: 144.5 ngl/uL
5. NdmB: 144.6ng/uL
6. NdmC: 146.1 ng/uL
7. NdmD: 104.8ng/uL
1.
15 uL vector 1
5 uL 10x NEB 4 Buffer
1 uL dpnI
29 uL H20
--
50 uL
2.
30 uL vector 2
5 uL 10x NEB 4 Buffer
1 uL dpnI
14 uL H20
--
50 uL
2e. Purification
Standard Qiagen PCR purification protocol. Elute in H20.
2F. Gibson cloning
1.
.86 uL vec 1
.51 uL ndmA_1
.50 uL NdmB
.40 uL NdmC
1.15 uL NdmD
15 ul 1.33x Gibson Master Mix
1.58 uL H20
--
20 uL total
2.
.86 uL vec 1
15 uL 1.33x Gibson Master Mix
4.14 uL H20
--
20 uL total
3.
.52 uL Vec 2
.51 uL ndmA_1
.50 uL NdmB
.40 uL NdmC
1.15 uL NdmD
15 ul 1.33x Gibson Master Mix
1.94 uL H20
--
20 uL total
4.
.52 uL vec 1
15 uL 1.33x Gibson Master Mix
4.14 uL H20
--
20 uL total
2g. Electroporation
1 uL desalted gibson reaction (2F1,2) ->50 uL electrocompetent BW25113-GuaB -> 1mL SOC -> 1hr incubation @ 37 deg
dilute 60 uL 2g transformations (1-2) -> 3mL Mineral M9 media + 34ug/mL chloramphenicol +/- 100mg Caffeine or Theophylline. Place on 30 deg shaker x 48 hrs
Result: construct enables growth (demethylation) on Theophylline. NdmC is not fuctional.
Streak 2j1 theophylline enriched culture onto LB-chloramphenicol plate for isolation of single colonies.
Pick 2 colonies from theophylline enriched streak for plasmid prep. Prep 5mL culture.
Result: both clones show anticipated ~5kB band corresponding to the complete operon.
 "
"