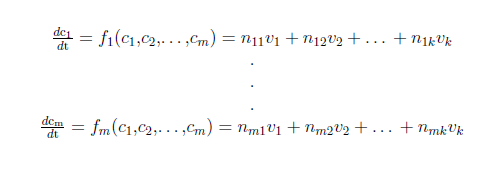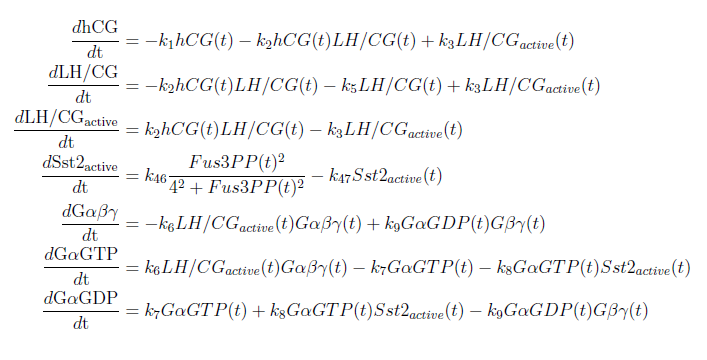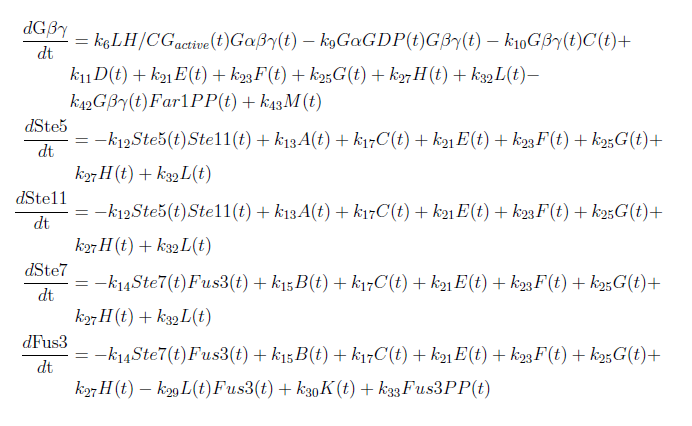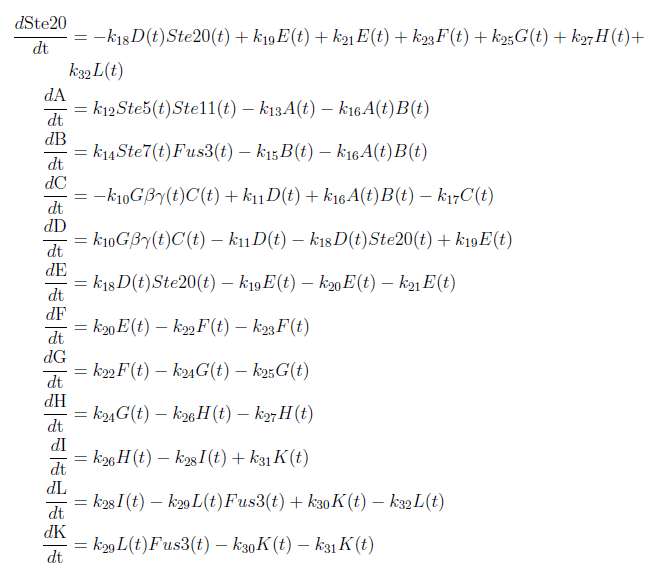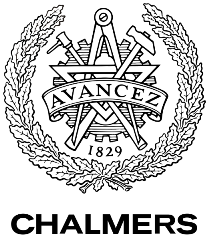Team:Chalmers-Gothenburg/Modelling
From 2012.igem.org
Modelling
Mathematical models are increasingly used to study certain aspects of signal transduction pathways, i.e. robustness against concentration variations [1], threshold properties and bistability [2] etc.
In an attempt to illustrate and understand the dynamics of our modified Saccharomyces cerevisiae (yeast) we created a complete mathematical model for the sequence of events when the hCG hormone binds to our modified receptor in the yeast (LH/CG receptor) and in the end starts an indigo production (click here for more information). Our model is based on the yeast pheromone pathway (click here for more information) [3].
The aim or goal of the model was to use it to analyze and understand where possible problems would occur in the lab and also to make some predictions, eg. estimates of how large concentrations of hCG is needed to, at least in theory, give an output signal, that is for indigo to be produced.
For molecules to enter yeast they must pass through a cell wall with limited permeability. Since hCG is a relatively large molecule and recent studies claim that it is too big to pass through the cell wall we also modeled the diffusion of hCG through the yeast cell wall. This to get some estimates if it is at all possible for the hCG to pass through to the receptor on the cell membrane with the cell wall intact or if it is necessary to delete the cell wall or at least increase its permeability.
Modeling the pathway of hCG in the modified yeast
For a biochemical reaction system it is common practice to use a set of ordinary differential equations (ODE’s) to describe the changes in the concentration of a biochemical species. In a system of m biochemical species with concentration ci (i=1,…,m) and k biochemical reactions with rates vj (j=1,…,k) you can write:
where nij denote the stoichiometric coefficients.
Our model was based on a complete mathematical model of the yeast pheromone pathway (see article [4]). By modifying this model to fit our project description we were able to discern some interesting results. The changes made are that instead of having the α-pheromone bind to the Ste2-receptor we have hCG binding to the LH/CG-receptor. Also, as mentioned in the project description, the Sst2 negative feedback regulator is taken out of the system along with the cell cycle arrest reaction.
With these modifications made from the original model we made a few assumptions for our model:
- The MAPK cascade will function as usual once the hCG has bound to the LH/CG- receptor.
- The cell cycle arrest can be deleted from the model without changing any other parts in the model since it has no feedback on the rest of the system.
- The rates used for the reactions (ki in the ODE system) are the same in our model as in the model for the yeast pheromone pathway with the exception of the hCG binding to the LH/CG receptor.
The modifications made to the model can be seen in figure 1 below.
Figure 1: Overview of the mathematical model for the modified yeast. With these modifications made the system of ODE’s for our model are as follows:
References
[1] Batchelor E, Goulian M. 2003. Robustness and the cycle of phosphorylation and dephosphorylation in a two-component regulatory system. Proc Natl Acad Sci USA 100: 691-696. [2] Levchenko A, Bruck L, Sternberg PW. 2000. Scaffold proteins may biphasically affect the levels of mitogen-activated proteinkinase signaling and reduce its threshold properties. Proc Natl Acad Sci USA 97: 5818-5823. [3] Bardwell L. A walk-through of the yeast mating pheromone response pathway. Peptides vol. 26, Issue 2, February 2005, p. 339-350. [4] Klipp E, Kofahl B. 2004. Modelling the dynamics of the yeast pheromone pathway. Yeast 21: 831-850.
</div>
 "
"