Team:Stanford-Brown/Protocols
Contents |
Methods
General Protocols
As provided by Jesse Palmer and Andrè Burnier
Media
Obviously there are many different media and recipes and you'll run across different ones for different purposes. Most of the time you'll autoclave the media after putting it together, although certain nutrients (vitamins, antibiotics) need to either be added after or require that the whole stock be filter sterilized instead of autoclaved. Any media recipe/protocol you find should tell you. Below we've given you the basics for two common media we use (you've probably heard of them)
LB
-Used for E. coli and B. subtilis
-Mix 20g/L of LB Broth powder into the desired volume of deionized water (which you can get in room 378, we'll show you how the filter system works)
-It is highly recommended that you mix liquid media in small aliquots (100-200μl) so the whole batch doesn't get ruined when one gets contaminated (LB is a very rich medium, this happens a lot)
BG11
-Used for Anabaena
-We have 50x stocks of BG11 in the fridge that are or can be aliquoted into 20mL samples
-Add 20mL of 50x stock to 980mL sterile water (autoclaved beforehand, and allowed to cool)
-This media doesn't have any solid carbon source, so it's pretty safe to make larger quantities
Antibiotic Stocks
We usually make our liquid stocks of antibiotics at 1000X, so that you add 1μl/mL to whatever media you are using. While the desired working concentration might change based on the plasmid, here are the stock concentrations we usually use for common antibiotics:
Chloramphenicol: 34mg/mL (100% EtOH)
Ampicillin: 100mg/mL (50% EtOH)
Kanamycin: 20mg/mL (H20 only)
Neomycin: 50mg/mL (H20 only)
Tetracycline: 15mg/mL (50% EtOH)
You'll want to make these by filter sterilizing; you cannot autoclave antibiotics. We typically store them in 1mL aliquots in a -20C or -30C freezer. Those stocks made with ethanol will not freeze and can be used put back in the freezer immediately. Those in water only will require thaw time.
Plates
Basics:
Using any typical media recipe, add 1.5% agar (15g/L) to the mixture before autoclaving after removing from autoclave. Let it cool until it is cool enough to hold for several seconds without hurting otherwise the media will be too hot and break down the antibiotic. Add the appropriate amount of antibiotic and pipette 15-20mL into each petri dish. Since the dishes come in sleeves of 25, it is usually good to make 500mL of the medium.
Keeping Them Sterile:
We have a UV hood that we usually make plates in. Gather everything you'll need to make the plates (empty petri dishes, pipette, pipette tip, sharpie, etc) and wipe down with 70% ethanol before placing in the hood. Sterilize for ~5-10min by exposing to UV lamp and make sure to turn off the lamp before putting media, antibiotics or any part of yourself into the hood.
Standard Molecular Workflow
N.B.: PCR is its own huge beast, so it's been given its own section following this one.
Liquid Culture
-inoculation: pipette tips work great for swiping or stabbing a colony
-media: LB works great for both E. coli and B. subtilis
-temperature: 37C works fine for both E. coli and B. subtilis
-antibiotics: if it is appropriate to select for the strain using antibiotics, add 1μl/mL stock solution for best results (but certainly not necessary):
1. pre-culture for ~6hrs in 20-25% final culture volume
2. incubate in container with capacity >200% culture volume, overnight
Cryostocking
Anytime you generate a new strain (i.e. transform a new combination of DNA parts) you should generate a cryostock for everyone to draw from in the future (in addition to miniprepping to generate a source of the DNA). It's pretty simple:
-In a cryostock tube (we'll point them out, but they're 1.8mL tubes with screw tops), mix a dense liquid culture of the strain with glycerol to the proper percentage (I think there's some flexibility but Jesse usually goes for 20% glycerol for both E. coli and B. subtilis) -So this might look something like: 800μl liquid culture + 200μl 40% glycerol solution Sterile technique is super important when making cryostocks
Miniprep (Qiagen, modified slightly)
1.Spin falcon tubes @7600rpm, @4C, for 5min to pellet
2.Discard supernatant by decanting
3.Reconstitute pellet in 250μl cold Buffer P1 and transfer to microcentrifuge tube 4.Add 350μl Buffer P2, invert 4-6 times to mix thoroughly
5.Add 350μl Buffer N3, immediately invert 4-6 time to mix thoroughly. This step will form a white precipitate.
6.Spin @13000rpm for 10min
7.Decant supernatant into spin column
8.Centrifuge 60sec, discard flow-through
9.Add 750μl Buffer PE to column, let sit for 60sec, spin 60sec
10.Discard supernatant and spin another 60s to dry
11. Transfer to clean microfuge tube and let sit 60s
12. Add 50μl EB and let sit 60s, spin 60s
13. Pipette and reapply flow through, sit 60s, spin 60s  14. Nanodrop
Nanodrop
The nanodrop machine is located in room 347 (ask us for the passcode), which is a shared lab and the nanodrop machine is a common good similar to the autoclaves, so make sure you are courteous to everyone in there. The actual nanodrop machine is a small white boxy thing with a raisable arm, to the right of the thinkpad computer at the back of the room. Before you go the room, make sure to bring a pipette that can measure 1μl, as well as an aliquot of PCR quality water or your specific elution buffer.
1. Unlock the computer
2. Lift the arm on the nanodrop and load 1 μL of your water/elution buffer aliquot onto the small, silver well (pedestal). Gently close the arm, then reopen the arm and dab the blank liquid off the machine with a kimwipe. Make sure you wipe off the metal nubbin on the arm of the machine, too. Put the arm back down. All this ensures that the nanodrop reading area is clean to begin with.
3. Open “Nanodrop 2000”
4. Select “nucleic acid” from the opening menu. Create a new file.
5. The nanodrop will perform a self calibration test; this will only takes a few seconds.
6. Select the appropriate type of nucleic acid you have in your sample from the dropdown menu. Most likely this is DNA, which is the default setting, but if you’ve got RNA you’ll need to select for it on there.
7. Next you need to run a blank. Repeat step 2., but while the arm is down click the "blank" button on the screen.
8. Load 1 μL of your sample. Gently close the arm and click the “Read” button
9. Let it read. If you clicked to create a new file in step 4, then it will ask you for your filename info and stuff like that. Go through that.
10. Finally, the results should pop up on the graph and the table below it. Make sure the graph has a good 260/280 ratio (usually greater than 1.75). The graph should have a pronounced peak in the left-center of the plot, and should be pretty low on the right side. Additionally, I think there is the beginnings of another peak at the very far left side of the plot, but it doesn’t matter. The curve of the graph should look relatively smooth. Generally, the quality of the read should be very high for something like a miniprep and will often be much lower when reading the product of a PCR or digest cleanup.
11. If the graph quality looks pretty good/normal, take note of the "ng/μl" value returned; this is the relevant information giving you the concentration of DNA in your sample
12. Repeat the scan (literally just click the "Read" button again) 2-3 more times to ensure that the read is consistent, and average the value
13. Save your data somehow (I just write it down, but you can screen capture if you want) and make sure to write the value on the tube containing the sample, wipe down the nanodrop, gently lower the arm, quit the program, and shut the computer. School’s out, you’re done!
Guidelines for Working with Enzymes and Reagents
Thaw reagents appropriately.
-Buffers, frozen antibiotics, etc should generally be thawed at 4C (the heater/chiller blocks we have work really well, and we don't have to worry about retrieving ice buckets all the time)
-Things like buffers and nuclease free water may be thawed at room temperature, but be sure to place at 4C as soon as thawing is complete
-Anything that is not water should be fully thawed and mixed gently before use to ensure proper ionic concentrations
Absolutely minimize time enzymes are out of the freezer by saving the enzyme(s) as the last reagent to add to the reaction and, when you do have to pull the enzyme out for use, making sure to keep in the small portable "bench top cooler jr" or heater/cooler block set to 0C or colder.
Digestion (adapted from openwetware Endy double digest)
-40 μl
-500-1000 ng DNA (as close to 1 μg as possible)
-1μl Enzyme 1
-1μl Enzyme 2
-4μl appropriate buffer (see NEB enzyme doubledigest finder; for any combination of the biobrick enzymes, buffer 2 or buffer 3 will be great)
-.4μl BSA
-top up w/ PCR H20
Mix reagents, adding enzymes last. Incubate at 37C for 1-2 hrs, then gel extract and clean w/ Wizard PCR cleanup kit Used for any combination of the EcoRI, XbaI, SpeI, PstI Store at -20C
Verification
Gel Casting
-.8% agarose (use if DNA > 1000bp)
-100 mL 1x TAE
-.8 g agarose
-(optional) .0283 g guanosine
-add if using gel for extraction (always make a fresh gel when using to extract DNA)
-1 aliquot (~5μl) gel red
Add dry agarose (and guanosine) to clean bottle (small enough to fit in microwave)  Add 100mL 1x TAE buffer and microwave with cap on but loose, swish periodically, until solution is clear and smooth
Pipette in gel red, directly into solution
Pour into gel tray, making sure that tray is oriented and tightly inserted such that leaks will not occur, and that the gel is level
Gel Loading & Running
Lane 1 should be ladder; use 1kb ladder or 100bp ladder depending on the size of your DNA samples
Digests require more (~1.5x) than the usual amount of loading dye
Gel Imaging (using Typhoon scanner)
Always scan a gel immediately after running Make sure the scanner area is clean; wipe ONLY with 70% ethanol and kimtech wipes Gel should be placed on scanner 'face-up.' That is, the wells should be oriented up, the same way the get is oriented in the gel box We'll do an in-lab tutorial for how to use the scanner and its program on the computer, easier to explain that way
Gel Extraction & Cleanup
Make sure to place gel on transilluminator face down (wells toward the glass) Remove as much excess gel matrix as possible without overexposing DNA to UV For cleanup, follow protocol for using the Wizard PCR Cleanup Kit, found below in "PCR" section Ligation (adapted from openwetware ligation protocol):
-10 μl
-30-50 ng vector DNA (closer to 50 is better)
->if insert is smaller than vector, 3:1 molar ratio, calc: [[(base pairs of insert)/(base pairs of vector)]*3]*(ng of vector) -1μl (1%) 10X T4 DNA ligase buffer ->if insert is comparable in size or larger than vector, 1:1 molar ratio
-.5μl (.5%) T4 DNA ligase
-top up w/ PCR H20
Always used digestion products that have been cleaned. As often as possible, use isolated inserts and vectors to avoid unwanted ligations If the reaction needs to be greater than 10μl, adjust amount of 10X ligase buffer and T4 DNA ligase so that they remain at 1% and .5% by volume,respectively. Incubate for 2hrs at room temperature (1hr is okay if necessary, but not as efficient), or overnight at 4C

Transformation (protocol from Kosuke)
-1 aliquot of NEB 5-alpha competent cells
-2-4μl ligation mixture
-250μl SOC media
1) thaw cells at 4C for 5 minutes
2) gently mix in ligation product
3) incubate at 4C for 20 min, meanwhile, heat SOC media to 37C
4) heat shock at 42C for 30 sec
5) return to 4C for 1 min
6) add 250μl pre-heated SOC
7) incubate at 37C for 1hr (shaking is not necessary), meanwhile, heat plates to 37C
8) plate, one plate w/ 100μl, one plate w/ 15μl
PCR (Polymerase Chain Reaction)
PCR vs. Colony PCR
There are a couple of different ways to introduce your template DNA, or the DNA you want to amplify, into a PCR mixture, and the method you use will depend on the source of the template DNA. The way I see it, there are four different scenarios:
1. Amplifying a genetic segment within a plasmid or isolated sample of DNA
This is the most basic scenario, where you have a tube of linear or plasmid DNA. This situation will definitely come up if you are trying to amplify a part from the registry directly, without going through the time-intensive process of transforming the part into e.coli and waiting for it to grow (quick note: you should go through the time-intensive transformation in parallel regardless, because you will need to replicate the part and make cryostocks of it, therefore saving it for later). In this case, you need first to know the concentration of your sample. If you don’t know it or it was not provided, you can learn the concentration for your sample by using the nanodrop machine located in room 347. It depends on the size of your template, but as a general rule, you need on the order of 25-50 ng template minimum for a successful PCR, so adjust the volume of your template in your PCR accordingly.
2. Colony PCR
You can also amplify both plasmid and genomic DNA straight from live cultures of organisms containing your desired sequence. You will usually have cultures in one of two forms: either in liquid culture, or spread on an agar plate. If you are amplifying from liquid culture, grow it up as much as you can and add 1μL of the culture to the PCR mix. If you're amplifying from the plate, there is no need to add a volume; instead, simply take a pipette with a pipette tip from the green box, gently touch the pipette tip to the desired colony on the plate (try to take as little from the plate as possible; agar can screw up PCRs), and then insert your pipette tip into the PCR mixture, pipette in and out to mix, and you’re done.
Polymerases and Supermixes
Platinum Blue PCR Supermix:
Platinum Blue is a 1.1X supermix, complete with dNTPs, buffer, and Taq polymerase. Taq is the polymerase that was isolated from extremophiles living in hot springs in Yellowstone, which you surely have all learned about in previous bio classes. Taq is the most pervasive polymerase used in molecular bio, and there are a host of downstream workflow kits and applications that are specifically designed to work with PCR amplicons produced by Taq polymerase (e.g. TOPO TA cloning kit). This specializations stems from the fact that Taq polymerase adds a single Adenine (A) base overhang on the 3’ ends of the amplicons; these overhangs need to be accounted for in subsequent ligation steps, and any ligation partner needs to have matching T overhangs to hook up with the Adenines.
For 20 μL rxn:
18 μL supermix
1 μL template DNA (assuming an adequate concentration)
.5 μL forward primer
.5 μL reverse primer
For 50 μL rxns:
45 μL supermix
1-3 μL template
1 μL forward primer (if using 1/10 dilution, this can be .7 μL)
1 μL reverse primer (if using 1/10 dilution, this can be .7 μL)
Add nuclease free water to 50 μL volume
rTaq PCR Supermix:
rTaq is a 2X supermix, meaning it needs to take up 50% of your reaction volume. It is inferior to platinum blue, as I think it produces more errors, and therefore it should not be used for accurate sequencing, or for any downstream application. That being said, it is really good for validation and testing to make sure constructs have made it into plasmids and stuff like that.
For 20 μL rxns:
10 μL supermix
1 μL DNA template (can be more if need be, since we have more leeway with rTaq and the requisite volumes of components)
.5 μL forward primer
.5 μL reverse primer
8 μL nuclease free water (or less, depending on what your volume of template is)
N.B.: This reaction can be scaled up to 50 μL rxns.
Phusion Polymerase:
Phusion is a high-fidelity, proofreading polymerase that absolutely flies: it is about 4x faster than your typical Taq polymerase you find in supermixes, and this speed will need to be accounted for in the Thermocycle conditions (see below). Also unlike Taq, Phusion produces blunt-end amplicons, and consequentially you may need to make adjustments to any downstream workflow plans (e.g. blunt end ligations, gibson cloning, etc.)
For 20 μL rxn:
4 μL 5x HF reaction buffer (I have never used GC buffer and don’t really bother with it.)
.4 μL 10mM dNTPS
.5 μL forward primer
.5 μL reverse primer
.5-14.4 μL template DNA
.1-.6 μL DMSO (OPTIONAL) - I use DMSO when I am doing a colony PCR sometimes, especially if I am redoing it because it didn’t work the first time. DMSO serves to relax genomic DNA, making it easier to read and amplify.
Nuclease water filled to 19.8 μL
.2 μL Phusion enzyme
N.B.: You will never add this much template but when putting together your own PCR mixtures and not relying on supermixes, you have a ton of leeway on how much template you can add, so long as it is suspended in water and none of that buffer EB crap that comes in the Qiagen kits. Never use that stuff, as it does have effects on the success of your PCR reactions.
Thermocycler Conditions
Taq polymerase (Platinum Blue and rTaq supermixes)
1. Initial Denature: 95 ̊C 2 min - The official Platinum Blue protocol calls for 94 ̊C for 3 min, use judgement as either will work.
2. Denature: 94 ̊C 15-30 secs - Use a shorter time if the amplicon is a relatively short segment of DNA, and a longer time if it is a relatively long piece of DNA.
3. Annealing X ̊C 15-30 secs - This is the most crucial step of the thermocycle! Your annealing temperature will be determined by the melting temperature of your primers. As a general rule, your annealing temperature should be about 5 ̊ lower than the lowest melting temperature of your primer pair. Additionally, if you are trying to add tails to your amplicon (e.g. you are trying to add restriction sites to the ends of your DNA template), you may need to drop the annealing temperature down even more. I have had primers with melting temperatures above 65 ̊ that needed to be annealed at 42 ̊. Additionally, if a primer may be difficult to anneal to the template, you can increase the annealing time for better results.
4. Extension 72 ̊ X seconds - Taq extension runs at 1kb per minute. Therefore, allowing the extension step enough time to fully copy the entire amplicon.
5. Repeat steps 2-4 32X
6. Final Extension 72 ̊C 5 min
7. Hold at 4 ̊C forever.
Phusion
1. Initial Denature 98 ̊C 30 sec
2. Denature 98 ̊C 10 sec
3. Annealing X ̊C 15-30 sec - Same principles of annealing with Taq polymerase apply here.
4. Extension 72 ̊C X seconds - Phusion is much faster than Taq, and requires 15-30 sec per kb. 5. Repeat steps 2-4 32X
6. Final extension 72 ̊C 5 min - You want to be a bit careful here; I wouldn’t run the final extension step for any longer than 5 min; Phusion is supposedly a very active enzyme, and I’ve been told it is best to not let it run for too long. Although I have never seen it happen, supposedly it can add random bases to the ends of amplicons.
7. Hold 4 ̊C forever.
N.B.: The standard protocols for various polymerases can be found at these addresses:
Platinum Blue Supermix: (http://tools.invitrogen.com/content/sfs/manuals/ platinumbluesupermix_man.pdf)
Phusion: (http://www.finnzymes.com/pdf/ f530_phusion_high_fidelity_dna_polymerase_prodinfo_low.pdf)
Primers
Designing Primers:
-Choose a forward and reverse primer from a location in the gene or plasmid that is sure to include the portion desired for amplification or sequencing
-For sequencing, it is desirable if possible to have primers that fall 50-150bp outside your desired region, to ensure that accurate reading occurs for the whole gene (often the first and last ~100bp in the read are very inaccurate). While for PCR, remember that the sequence portion corresponding to the primers themselves will be amplified, and also primers should normally be between 15-30bp in length (around 20bp is ideal) -desired melting temperatures are generally between 55-65C as you will see, melting temperature is a function of length and GC content, so it is often difficult to design primers in regions much greater than 50% AT. Forward and reverse primers should have the same melting temperature, or with a difference of no more than 2 degrees.
-The annealing temperature used for a pair of primers should be set at 5 degrees below the lower melting point of the primer pair. Using a tool like ApE or Geneious makes it easy to select certain sections of a sequence to check for primer features like melting point and GC content. IDTs 'Oligo Analyzer' is a great tool to check for primer dimerization, hairpin structures, etc. (http://www.idtdna.com/analyzer/Applications/OligoAnalyzer/). Use this tool or something like it as a final check to make sure your primers will not be likely to react with themselves or each other around the temperatures they will be active for gene interaction
-NCBIs Primer Blast is another great tool. It can be used both to help design the primers and to ensure that the primers you choose will not amplify any genomic DNA in a colony based amplification (http://www.ncbi.nlm.nih.gov/tools/primer-blast/)
Ordering Primers:
-All primer orders are from ELIM Biopharm (http://elimbio.com/)
-Usually any primers you order during normal work hours will arrive next day
Primer Dilution (stock preparation):
-Once you receive your primers, you need to dilute them; They can be dilutions can be made 1/10 or 1/20, both work well as long as you keep track and adjust your reactions appropriately.
-Typically we create 100-200μl working stocks; it will take a long time to use up that much primer
PCR Cleanup (using Wizard SV Gel and PCR Purification System)

Sample Prep
A. Gel Extraction:
- Following electrophoresis, excise DNA band from gel and place gel slice in a 1.5ml microcentrifuge tube.
- Mass gel slice (by massing the tube containing the slice and subtracting the mass of an empty tube)
-Add 10μl Membrane Binding Solution per 10 mg of gel slice. Vortex and incubate at 50–65°C until gel slice is completely dissolved (usually 10-15 minutes)
B. PCR Amplifications:
-Add an equal volume of Membrane Binding Solution to the PCR amplification.
Binding of DNA
1. Insert SV Minicolumn into Collection Tube.
2. Transfer dissolved gel mixture or prepared PCR product to the Minicolumn assembly. Incubate at room temperature for 1 minute.
3. Centrifuge at 12,000 × g for 1 minute. Discard flowthrough and reinsert Minicolumn into Collection Tube. If you are worried about the final concentration of your purified product, you can repeat this step to maximize the amount of DNA bound to the filter.
Washing
4. Add 700μl Membrane Wash Solution (ethanol added). Centrifuge at 12,000 × g for 1 minute. Discard flowthrough and reinsert Minicolumn into Collection Tube.
5. Repeat Step 4 with 500μl Membrane Wash Solution. Centrifuge at 12,000 × g for 5 minutes.
6. Empty the Collection Tube and recentrifuge the column assembly for 1 minute with the microcentrifuge lid open (or off) to allow evaporation of any residual ethanol.
Elution
7. Carefully transfer Minicolumn to a clean 1.5ml microcentrifuge tube.
8. Add 30-50 μl of Nuclease-Free Water to the Minicolumn. Incubate at room temperature for 1 minute. Centrifuge at 12,000 × g for 1 minute. By adding less water, like 30 μl, you will increase the concentration but decrease the total amount of product. On the flipside, if you want to maximize product, you can maximize elution volume so long as you don’t care about concentration.
N.B.: you can also increase yield by warming the elution water before hand. I usually warm it to 40 ̊C with good results.
9. Discard Minicolumn and take sample to nanodrop (see 'Nanodrop', below) 10. Store DNA at 4°C (temporary, ~few weeks) or –20°C (long-term).  The standard protocols for the SV Wizard Gel and PCR purification kit can be found here: (http://www.promega.com/resources/protocols/technical-bulletins/ 101/wizard-sv-gel-and-pcr-cleanup-system-protocol/)
Hell Cell
Cold Assay 9-13-2012
1. Grow up 5ml cultures of negative control, betab, and otsab (one each) from 5ul of old culture and 5ml of new LB + Amp.
2. Allow cultures to grow for 24hrs
3. Dilute by putting 20 uL into fresh 5 mL of LB+Amp. Take OD600 of these diluted cultures using spectrophotometer.
4. Prepare six 8-tube PCR strips: 2 for blanks of LB+amp, 2 for negative control, 2 for betAB. Pipette 175 uL of each culture into the appropriate tubes.
5. Place strips in PCR machine and set on 15°C forever.
6. Open the lid of the PCR and take of caps of all the tubes daily for a few minutes to allow for air passage
7. About a week later, transfer contents of strips into 96-well plate and use spec to take OD 600 reading.
Desiccation Assay 9-13-2012
1. Grow up 5ml cultures of negative control and betAB (one each) from 5ul of old culture and 5ml of new LB + Amp.
2. Allow cultures to grow for 24hrs
3. Use the mini plates the one about 5cm across. 60 plates in total (three samples for betAB and one sample for negative control) Label plates 15 plates neg, 15 as mntH, 15 as ots, and 15 as bet.
4. Place sample into plates. 300ul of corresponding culture into each plate.
5. Swirl dish around so media spreads evenly across bottom.
6. Place plates in incubator for 24 hours. Don’t parafilm or foil or wrap. Tap on sides to keep them closed is fine.
7. After 24 hours, the plates should be dry. Place 1ml of fresh LB (no amp) into the dish. Then spot 10^-2, 10^-3, 10^-4. Same method of 900ul of LB in tube and dilute 100ul from each previous tube. Spot 10ul spots. You can also do this using the multichannel pipette. There are only 2 loading boats though, so you need to wash with ethanol between samples. I did a volume of 180 fresh LB in each well and moved 20ul from each previous sample over. My recommendation is to try to minimize the time spent in the new 1ml fresh LB before spotting.
8. Incubate spot assays at RT wrapped in foil at 37C overnight
9. Count results of spot assay next day.
Protocol UV-C survival assay E. coli
Revised 8-21-12
1) Grow E. coli over night in LB;
2) In the following morning (8 to 9am), inoculate 50 uL in 2ml of new LB medium (1:40 dilution);
3) Setup the experiment. Fill enough microfuge tubes with 900 ul sterile saline solution (0.9% NaCl) for the whole experiment;
3) After 4 to 6 hours, transfer 1 ml into a sterile microfuge tube and
4) Centrifuge at 9,000 rpm for 3 min;
5) Discard supernatant, add 1 ml saline solution, ressuspend by pipetting and vortex;
6) Repeat steps 4 and 5 once and then go to step 7;
7) Make two serial dilutions 1:10 (10E-1 and 10E-2). Use a microfuge tube filled with 900 ul saline solution and transfer 100 ul of the original cell suspension. Vortex and transfer 100 ul of the resulting dilution to another microfuge tube filled with 900 ul saline solution and mix by vortexing;
8) Transfer 10 ul aliquots of each dilution to the hemacytometer and proceed with cell counting;
9) Adjust the cell concentration to 10E7/ml to a final volume of 5 ml saline solution in a glass Petri dish;
10) Adjust the flux of the UV lamp to 1.2 J/m2/s;
11) Before start the irradiations, transfer 100 ul of the cell suspension into a microcentrifuge tube containing 900 ul saline solution (already 10E-1 dilution). This will be the non-irradiated control;
12) Proceed with the exposures times: K12: 0s (control), 2s, 5s, 10s, 20s, 30s, After each exposure time, transfer 100 ul of the irradiated cell suspension into a microcentrifuge tube containing 900 ul saline solution (already 10E-1 dilution).
13) After finishing the irradiations, for each dose proceed with the serial dilution (10E-2, 10E-3, 10E-4, 10E-5) using microfuge tubes filled with 900 ul saline solution.
14) Inoculate 10 ul of each dilution on LB-agar plates. One plate per dose, divided in 6 sections.
15) Incubate at 37C over night.
16) In the following morning, proceed with colony counting and put the results in a graph showing a survival curve
Acid Base Assay 9-24-2012
1. Grow up 5ml cultures of negative control, recA, dps, sdaB (one each).
2. Allow cultures to grow up overnight.
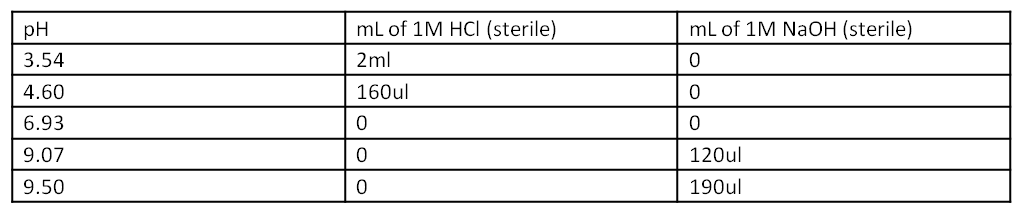
3. Set up acid and base solutions first. Fill 5 tubes with 10ml fresh LB and 50ul amp. Label: ph3.54, ph4.60, ph6.93, ph9.07, ph9.50. The HCl and NaOH have been sterilized in 50ml tubes. They are above the gels in the cabinets. They must always be stored in separate secondary containers. Eye protection is recommended. Glasses are above where we keep tip boxes. Acids and bases can go down the drain, just make sure there is plenty of running water. Any acidic or basic LB can go in liquid waste.
4. Verify 5ml of each acidity with pH meter.
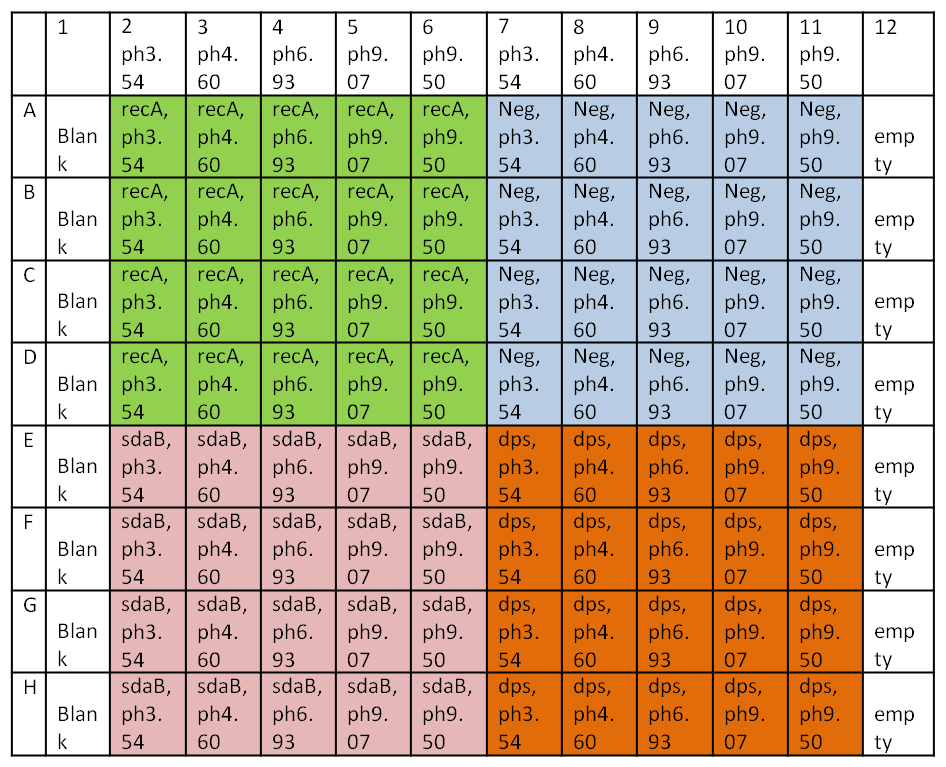
5. Set up acid base 96 well plate. Each well contains 180ul of appropriate pH media and 5ul of cells.
To summarize, the different color regions are different genes. The easiest way to do this is to fill 180ul of media into the right columns with the multichannel. Then put in 5ul of right cells. Column 1 is a blank which is 180ul LB + amp. Columns 2 and 7 are ph3.54 media, Columns 3 and 8 are ph4.60, Columns 4 and 9 are ph6.93, Columns 5 and 10 are ph 9, and Columns 6 and 11 are ph9.50.
6. Load plate into spec. Setup the spec using the computer. There should be a template from before saved in the iGEM folder on desktop. Make sure to set the spec to 37oC. Take the OD at 600 every 5 minutes for 24 hours. Shake the plate for 1 minute between reads and for 1 minute before each read. You need to specify which wells are blank. Also, in order for data to be collected, you need to label the wells and give them a name.
7. Let the spec run for 24 hours. After the 24 hours, take the data off the computer. Use the vacuum waste to clean up each well, and toss the plate in biohazard.
Venus Life
Construct Development
Potential cell-cycle dependent promoters in E. coli and their sequences were identified from literature (Quiñones 1997, Sun 1994, Ogawa 1994). Primers were designed to isolate these sequences and add on BioBrick cut sites according to the Silver lab method (Phillips 2006). Colonies of E. coli were selected from plates and sequences were isolated using colony PCR. Sequences were verified using BioPharm oligo sequencing and gel electrophoresis. PCR product was digested for 2 hours at 37C with EcoRI and SpeI. Digestion products were isolated via gel extraction; two samples were ligated with pSB1C3 cut with EcoRI and SpeI, and BBa_E0840 cut with EcoRI and XbaI, respectively. Ligation products were transformed into NEB-5a competent cells via heat-shock; promoter-pSB1C3 strains were plated then inoculated in LB+Chloramphenicol and promoter-E0840 strains were plated then inoculated in LB+Ampicillin.
Bulk Assay
50mL cultures of promoter-E0840 were grown overnight in 125mL flasks with LB+Amp, and some culture was diluted into 2 15mL samples with OD600 between 0.1 and 0.3. To synchronize cell cycle, serine hydroxamate (SHX) was added to both replicates to a final concentration of 10mg/mL and allowed to incubate at 37C for 1.5 hours (Ferullo 2009). After incubation, OD600 was taken to verify cell-cycle arrest. Samples were pelleted and resuspended in LB+Amp and replaced in the 37C shaking incubator.
Every 2.5 minutes, 1mL sample was removed from each replicate and OD600 was measured. The same sample was transferred into a microcentrifuge tube, pelleted, aspirated, and resuspended in M9. This sample was then measured in the fluorometer.
Microscopy Assay
4mL cultures of promoter-E0840 were grown overnight in 15mL conicals with LB+Amp. At an OD600 reading between 01. and 0.3, 40uL of 100X SHX was added to synchronize cell cycle. After 90 minute incubation, cells were pelleted and resuspended in LB+Amp without serine hydroxamate. 10uL of sample was pipetted onto a glass slide then covered with an agar pad. A timelapse was taken for 2 hours, taking images every 5 minutes.
Biomining
Flagella Removal Protocol
Procedure from Westerlund-Wikstrom Paper Cited
For large scale removal and purification of the flagella, we hoped to adopt the purification method stated in the paper entitled “Functional expression of adhesive peptides on flagellin”. The method outlined involved four steps:
1. Shearing of flagella from bacteria
2. Separating the cells from flagella
3. Purifying the flagella
4. Detection of the flagella
In the first step, agar plates of the bacteria were collected in Tris-HCL buffer. The cells then had their flagella sheared off by a Turrax homogenizer at 20,000 r.p.m. The next step included pelleting the cells, and re-centrifuging the supernatant to ensure that all residual bacterium were removed. The supernatant, which contained the flagella, was then ultracentrifuged to form a pellet which was reconstituted in 1ml 10mM Tris-HCL and 0.5% deoxycholate. The third step then involved purifying the flagella. This was accomplished by isopycnic ultracentrifugation in a 10-60% sucrose gradient in Tris-DOC. The complete conditions for ultracentrifugation are outlined within the article. Concentrated light was then used to illuminate and detect the flagella, which were then collected and dialysed against Tris and distilled water.
Once the flagella have been collected, we would need to separate the metal ions for collection and use. The purified intact flagella (complete with metal binding peptides and metal ions) would be collected on Gelman cellulose acetate filters. The Tris-DOC buffer would be washed through with 0.5M KCL. The filter and flagella were then immersed in 5M urea - 0.05M KCL for half an hour at 26 C. At this point, the filaments (flagellin) dissociated while the hook-basal body complexes that make up the rest of the flagella were eluted.
 "
"