Team:MIT/ResultsFoundational
From 2012.igem.org
DEPRECATED. DO NOT USE OR EDIT. If a page uses this template, relink with MIT-results2.Introduction
Paragraph tying our literature search to our design process to our two testbeds for Strand Displacement.
Link to Primer on Strand Displacement
Design
Placeholder for high level graphic:

Iteration 1
The first iteration aims to replicate existing DNA strand displacement using derived RNA strands.
We used the DNA S6 domain and toehold sequences used by Qian et al. 2011, and converted them into RNA, something that has not been published on. Furthermore, we added 2'O Methyl modifications the ribose of each base. This is a widespread RNA modification (e.g. Behlke et al. 2008) to protect it from its own catalytic 2'OH, which can catalyze backbone cleavage. It is also beneficial, as a modified base can protect the strand from enzymatic cleavage by rendering it to a non-substrate.

Regular RNA ribose (left) vs. 2'O-methyl ribose (right). Source: Wikipedia.
Iteration 2
In the second iteration, we optimized our sequences for an in vivo environment.We set out to design new sequences that:
- have increased thermodynamic stability (greater Tm, the melting temperature of a double-stranded complex)
- are orthogonal to RNAs expressed in HEK293 cells
- are more protected from nuclease activity
- are more protected from the RISC complex
We achieved this by:
- increasing the domain and toehold lengths
- increasing the G/C content
- analyzing the HEK293 transcriptome
- researching literature for protective modifications
Sequence Selection Algorithm
We analyzed HEK293 transcriptome data by first fetching the accession IDs from HEK293 Cell Database and then the corresponding sequences from GenBank. Duplicate sequences were removed.
We first chose an 8 nucleotide (nt) toehold sequence. The sequence space was restricted to a 3-base code (A/T/C) with the sequence ending in 5' CA 3' (clamp, see Qian et al. above). We calculated the Hamming distance between each potential toehold and the transcriptome, along with the number of exact consecutive nucleotide matches from the beginning to rank each potential sequence. The resulting list can be sorted by deviation from the mean for each possible Hamming distance (0-8) and exact consecutive run (0-8). The best sequence would be one for which low Hamming distance instances are relatively (>=1 standard deviation) less common and high Hamming distances are relatively more common. Similarly, shorter consecutive run instances are more favored over longer ones.
5' UACUAC CA 3' // has repeats
Top 5 generated toeholds.
5' ACUAUC CA 3' // choose this one
5' CCUAUA CA 3'
5' CUAUAC CA 3'
5' ACCUAU CA 3'
Then, the same approach was used to identify the 20 nt domain sequence. However, generating all sequences is very time intensive with roughly 320 combinations of 3-base code sequence to evaluate. Instead, a genetic algorithm was used to generate sequences, evaluate them and to improve on the top scoring sequences by mutating them or performing crossovers with other sequences. After an overnight run of four threads of this algorithm, the best sequences were selected. Then the sequence with the highest G/C content and fewest repeats was chosen. In principle, the latter constraints can be added to the automatic scoring feature of the genetic algorithm.
Protective Base Modifications
The original strands were modified with 2'O-Methyl groups on the ribose sugar (see above). We kept these modifications on the newly designed strands.
For strands without 3' fluorophore (ROX or Alexa 488) modifications, we added a phosphate group to the 3' end. According to IDT, this protects from some 3' exonuclease degradation. Input strands received an IR800 modification on the 5' end for quantification and potential protection from 5' exonucleases.
Protective Sequence Modifications
To protect against the RISC complex, we made a variant of the reporter top strand that included a bulge 10 nucleotides from the 3' end (Iba et al., 2012). There were 3 different bulges published by Iba et al., but only one of them, 5' CACU 3', used a 3-base code, so we chose this sequence as the bulge.
Nucleic Acid Delivery

In order to implement RNA strand displacement cascades in vivo, we first demonstrated our ability to deliver nucleic acids to mammalian cells through a variety of methods and reagents. In this section, we will review three different nucleic acid delivery techniques to mammalian cells, (1) Delivery of plasmid DNA, (2) Delivery of 2'-O-Methyl RNA and (3) Delivery of plasmids with inducible protein control.
1. Delivery of Plasmid DNA to Mammalian Cells
Through the Gateway method, we have assembled many promoter-gene constructs as detailed on our Biobrick Parts Page. After construction of the plasmid, we delivered the plasmid DNA to HEK293 human embryonic kidney cells through transient transfection. In particular, we used lipofection with Lipofectamine 2000 reagent. See Materials and Methods for our Gateway and lipofection protocols.
Images of eYFP/mKate transfection from Confocal
This figure shows HEK293 cells which were transiently transfected with Hef1A:eYFP and Hef1A:mKate using Lipofectamine 2000. Equimole amounts of DNA were delivered, 500 ng total per 1.65 uL of reagent. Images were taken on a Zeiss microscope at 10X.
2. Delivery of 2'-O-Methyl RNA to Mammalian Cells
The movie above shows HEK293 cells expressing constitutive eYFP with a 2'-O-Methyl RNA strand labeled with ROX (5-carboxy-x-rhodamine) on the 3' end. As time passes, the complex/vesicles are uptaken by the cell, releasing their payload resulting in whole cell fluorescence. Each frame is 5 minutes, movie encompasses 200 minutes in 9 seconds.
 Time point images taken at t = 0, 2, 3, and 4 hours post-transfection. Images taken at 10X on Zeiss microscope.
Time point images taken at t = 0, 2, 3, and 4 hours post-transfection. Images taken at 10X on Zeiss microscope.
Spontaneous dissociation and rapid degradation of RNA oligomers often occur under in vivo conditions. Our experiments required that our RNA be stable and react predictably in cells. Thus, we chose to use chemically-modified 2'O-methyl RNA, a naturally occurring and nontoxic RNA variant found in mammalian ribosomal RNAs and transfer RNAs. The -OH functional group at the 2' position on each nucleotide is replaced with an -OMe group, which prevents cleavage of the RNA backbone. In addition to a significant increase in stability against nucleases, the modification seems to confer increased melting temperature. This minimizes the probability that the RNA strands will dissociate upon introduction to the cellular environment.1. Because 2'O-methyl RNA exhibits different chemistry than normal RNA, we developed specific protocols to deliver these constructs to mammalian cells.
 Figure 1
Figure 1
Once we demonstrated ability to deliver 2'-O-Me RNA to mammalian cells, we ran optimization experiments to optimize the ratio of 2'-O-Me RNA delivered to RNAiMAX (transfection reagent used) to achieve maximum transfection efficiency. The invitrogen protocol2 for Lipofectamine RNAiMAX reagent recommends 0.6 to 30 pmol of nucleic acid delivered per well per 24-well plate. Throughout our experiments we observed that we could see high transfection efficiency for 30 pmol of RNA but almost no transfection for 15 pmol, so we ran a titration experiment, (Figure 1), testing out 20, 25 and 30 pmol of RNA with 1.0, 1.33 and 1.5 uL of transfection reagent and see saturation levels at 25 pmol. Therefore, the rest of our experiments were standardized around 25 pmol of RNA delivered with 1.33 uL of reagent:
Figure 1 shows a normalized graph of flow cytometry data with the FITC channel on the x-axis showing arbitrary fluorescence units and cell count on the y-axis, normalized. We can see that as we move from 0 to 20 to 25 picomoles of double stranded RNA delivered, the entire cell population shifts toward higher fluorescence levels, but we start to saturate the system as we see fluorescent population shift left at 30 picomole of RNA delivered.
3. Inducible Control of Protein Expression
 In this circuit, a constitutive mammalian promoter, Hef1A, drives expression of a trans-activator, rtTA3 which activates the Tre-Tight promoter, in the presence of a small molecule DOX, which drives expression of a fluorescent red protein, mKate. In addition, a plasmid containing the Hef1A promoter driving expression of a blue fluorescent protein, TagBFP, is used as a transfection marker
In this circuit, a constitutive mammalian promoter, Hef1A, drives expression of a trans-activator, rtTA3 which activates the Tre-Tight promoter, in the presence of a small molecule DOX, which drives expression of a fluorescent red protein, mKate. In addition, a plasmid containing the Hef1A promoter driving expression of a blue fluorescent protein, TagBFP, is used as a transfection marker
After demonstrating the ability to transfect all of the RNA parts necessary for strand displacement, the next step to scaling further in vivo is having the cell transcribe our RNA parts. Specifically, to characterize strand displacement, we wanted to have control over the input strand. To do so, we needed to test an inducible expression system and demonstrate control over the selected output. Here, we have rtTA3 being constitutively expressed by the Hef1a promoter. With the presence of doxycycline (DOX), rtTA3 can activate the TreT promoter, and express protein (mKate in this case).

100,000 HEK293 cells were transfected with equimolar rations of Hef1a:rTTA3, TreT:mKate, and Hef1a:TagBFP (as a transfection marker), standardized to 500 ng total plasmid DNA, with 1.65 uL Lipofectamine 2000. After 48 hours, cells were imaged on a Leica Confocoal Microscope at 10x. We can see that we have transfection, since cells are fluorescing blue. Also, we can see that as we increase the concentration of DOX present, we see an increase in red fluorescence.

100,000 HEK293 cells were transfected with equimolar rations of Hef1a:rTTA3, TreT:mKate, and Hef1a:TagBFP (as a transfection marker), standardized to 500 ng total plasmid DNA, with 1.65 uL Lipofectamine 2000. After 24 hours, DOX was then added to 16 different concentrations ranging from 0.1 nM to 5000 nM. Lastly, cells were harvested for flow cytometry after 48 hrs and allowed to count 10,000 events. As we increase concentrations of DOX, the mean red fluorescence increases.
In Vivo RNA Strand Displacement
Overview Graphic:

PLACEHOLDER - LINKS
Design One
In vitro
To inform our in vivo work, we verified our designs in vitro (see Protocol). Even though the sequences were from previously published work, reactions with RNA inputs to RNA reporters or other gate:output complexes has not been published, so we needed to establish that RNA works as the substrate for toehold-mediated strand displacement.
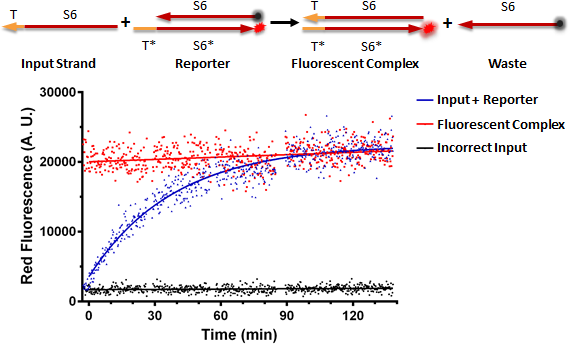
Testing an RNA reporter in vitro. The negative control received a scrambled input, whereas the positive control only contained the fluorescent complex. The experimental well contained the reaction shown at the top of the figure, where the toehold T of the input strand binds the complementary toehold T* on the reporter complex, which is non-fluorescent due to the presence of a quencher near the fluorophore. This results in the initiation of branch migration, which results in a fluorescent complex, and a waste molecule (free quencher strand).
Conclusion: RNA can be used as a processing medium instead of DNA.
In Vivo Interation One: Lipofectamine 2000 Transfection of RNA version of Reporter from Winfree/Qian 2011 Paper
Our first strategy to implement RNA strand displacement in vivo was to adapt the DNA sequences of inputs, gates and reporters from the Qian/Winfree, "Scaling Up Digital Circuit Computation with DNA Strand Displacement Cascades," 2011 Science Paper to 2’-O-Methyl RNA strands to transfect into mammalian cells. . In the first foundational experiment, HEK293 (Human Embryonic Kidney cells) were used that constitutively expressed a yellow fluorescent protein (eYFP) in order to be easily visible in microscopy images. 200,000 HEK293 cells were seeded into four wells of a 24 well plate in supplemented DMEM without phenol red pH indicator. The negative control well did not receive any RNA. As a transfection reagent, each well received 1 uL of Lipofectamine 2000. The positive control well received 5 pmol of a gate strand tagged with a ROX fluorophore annealed to an input strand, to act as a product of a strand displacement reaction. The scrambled input well received 5 pmol annealed double stranded reporter with quenched ROX along with a 5 pmol of an input strand containing the correct toehold domain but the incorrect binding domain. Therefore, when both constructs are inside the cell, a strand displacement reaction should not occur, and the fluorophore remains quenched. In the final well, correct input, the cells received 5 pmol of double stranded reporter as well as 5 pmol of an input strand with the correct toehold domain and hybridization domain. Accordingly, we should expect that the toehold of the input strand binds to the complementary exposed toehold on the double stranded reporter, and will branch migrate and effectively kick off the output strand of the reporter that is tagged with a quencher. Therefore, the fluorophore will no longer be quenched, yielding red fluorescence.

Images taken at 15.5 hours post transfection on a Zeiss microscope at 10X.
However, we see from the above figure that in the negative control well, 200,000 HEK293+eYFP cells are healthy and adherent. In the positive control well, we see localized red fluorescence in the form of vesicles as well as distributed, whole cell red fluorescence. In the scrambled input well, we see red vesicles as well as red whole cell fluorescence. In the correct input well, we see only whole cell red fluorescence.
Conclusion: The reporter comes apart in the vesicles and possibly inside of the cells, too.
In Vivo Interation Two: Switch Transfection reagent to RNAiMAX
From the first foundational experiment, we observed localized red fluorescence in vesicles as well as whole cell fluorescence. This indicates that our reporter complex is either melting, being degraded, being recognized by a specific enzyme etc as well as the reporter is coming apart inside of the lipofectamine vesicles. We researched better transfection reagents for double stranded RNA, and found that Lipofectamine RNAiMAX is designed specifically for the delivery of double stranded RNA, whereas Lipofectamine 200 is specifically designed for the delivery of plasmids.
Once we received the new transfection reagent, we set up experiments similar to the initial experiment but with an optimized protocol for RNAiMAX (See Materials and Methods).

These images were taken on a Zeiss microscope at 10 X. The row labeled Reporter received only our double stranded quenched ROX complex with an exposed toehold. We can see that as the time goes from T=0 minutes post transfection to T=60 (24 hours post transfection) the reporter is once again either being degraded or melting inside of the HEK293 cells. We see an interesting trend in the row labeled incorrect input. This well received the reporter complex along with our input with the correct toehold but the incorrect binding domain. It appears that the toehold of the incorrect input can bind to the exposed toehold of the reporter, somehow stabilizing the complex. Finally, in the well that received the correct input for a strand displacement reaction along with the reporter, we see identical results to the just reporter well, which makes our argument for strand displacement initially unclear. The success of this experiment, however, is the fact that we no longer observe red vesicles, which indicates that RNAiMAX is indeed a better transfection reagent.
Conclusion: RNAiMAX is a superior reagent for double stranded RNA, and we now only see the reporter coming apart inside of cells instead of inside vesicles.
In Vivo Iteration Three: Tag RNA strand with an Alexa Fluorophore to act as a transfection marker
Since we continually observed a trend of the reporting strand coming apart inside of the cell (i.e. the quencher no longer quenching the fluorophore) we modified the reporter with an additional fluorophore, AlexaFluor488 to act as a transfection marker, so that we could observe the reporter entering the cell. AlexaFluor488 is a green dye, and we predict that if the two strands melt inside of HEK293 cells then we would see an initially green vesicle enter the cell and then localized green and red fluorescence in the cytoplasm, indicating that the strands came apart.
Nice Data showing reporter melting inside cell from 7/29 powerpoint
Design Two
In vitro
To verify that the modifications we made in the second iteration of our strand design (see above) do not interfere with strand displacement, we tested them in vitro.

Testing the second iteration of an RNA reporter in vitro. Compared are the strands without bulge (left) and with the protective bulge (right). Kinetics and completion levels are nearly identical between the two variants, suggesting that the bulge does not interfere with strand displacement.
Conclusion: The 2nd set of de novo designs work for strand displacement and the bulge does not significantly affect the toehold-mediated strand-displacement reaction.
In Vivo Data:
Nucleofection Data + description
Transfection Data + description
 "
"
