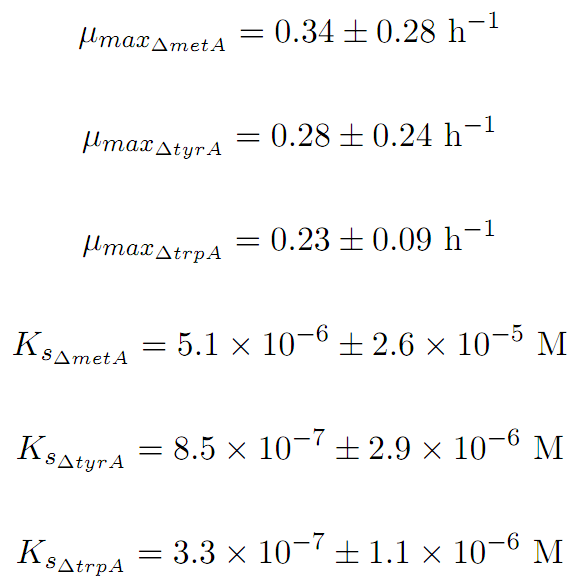Team:British Columbia/Consortia
From 2012.igem.org

Consortia Model Description
The three interdependent E. coli auxotrophs studied in our project are ΔtrpA, ΔmetA, and ΔtyrA. In order to motivate how to set up our co-culture experiments, we wanted to model the population dynamics
This model is designed to predict the consortium’s population dynamics, based on the measured amino acid excretion rates without tuning them. However, the code of the model is written in a way that when we decide to tune the population by adding in different concentrations of inducers, we can simply update the excretion and consumption rate equations. Thus this model is valid for both situations. It can even be generalized to be used by any interdependent E. coli consortium when Trp, Met, and Tyr are the limiting nutrients/substrates for their growth, with some modifications.
The key assumption for this model is that the Tryptophan, Methionine, and Tyrosine are the only growth limiting substrates for TrpA-, MetA-, and TryA- E. coli knock outs respectively.
Monod equation is the most commonly used model for microbial growth and it can reasonably fit a large variety of data empirically[1]. Also, it shares the key assumption described above. Thus it is decided to be one of the fundamental governing equations for this model.
For those who are not so familiar with the Monod equation, its key equation states like this:
where µm is the maximum specific growth rate, S is the limiting substrate concentration, and Ks is the concentration of the limiting substrate when the specific growth rate is half of the maximum (Ks=S when µg=1/2µm) [1]. Ks has the name of saturation constant or half-velocity constant[1].
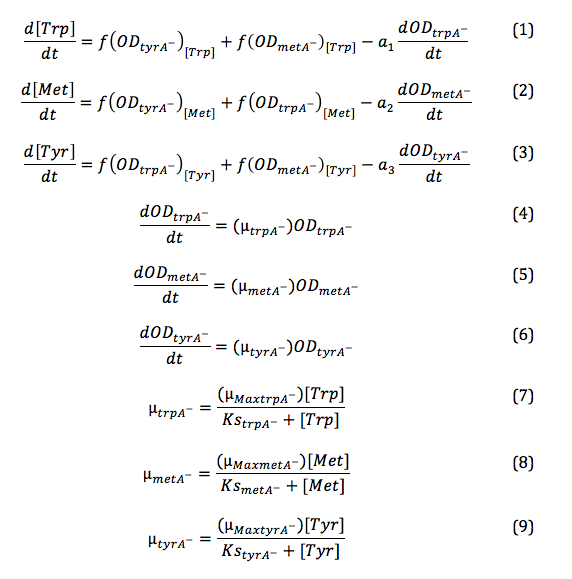
Equations:
Constants:
Some of the key constants for the Monod Kinetics are obtained through analyzing various growth rates under different known amino acid concentrations. The values are listed as follows:
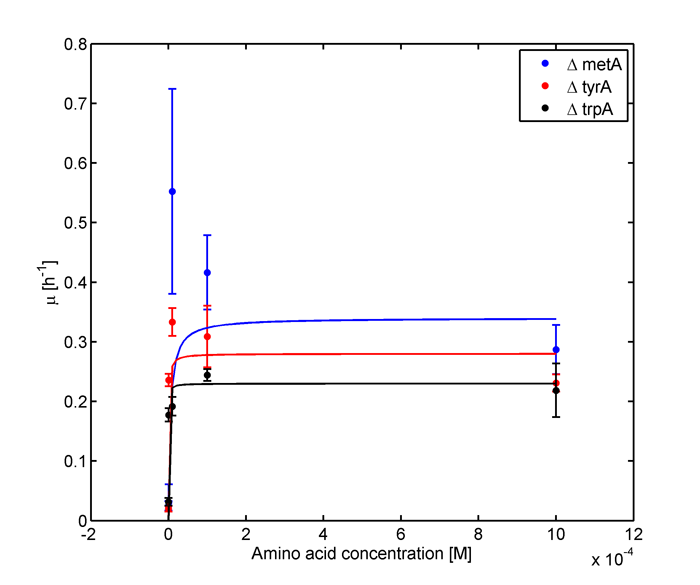
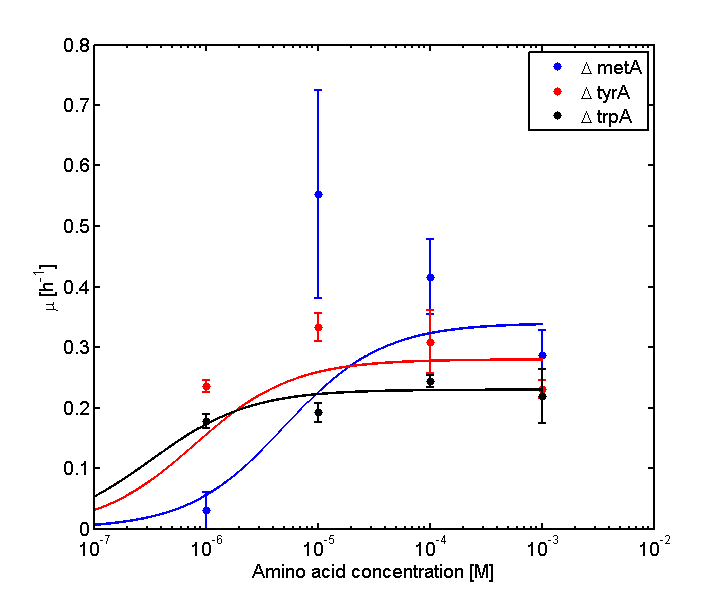
and its wet lab data used is mainly based on the following graphs:
Figure 1: Monoculture Growth Rates at various Limiting Amino Acid Concentrations
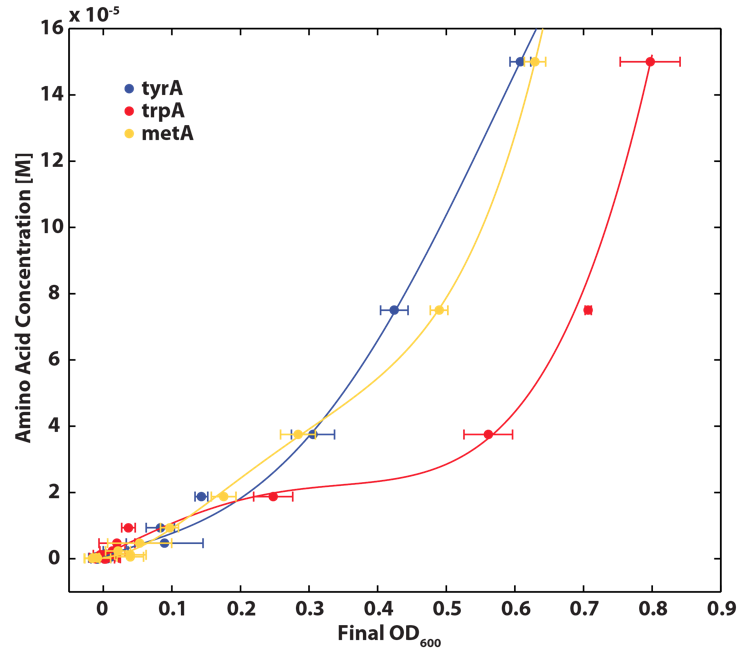
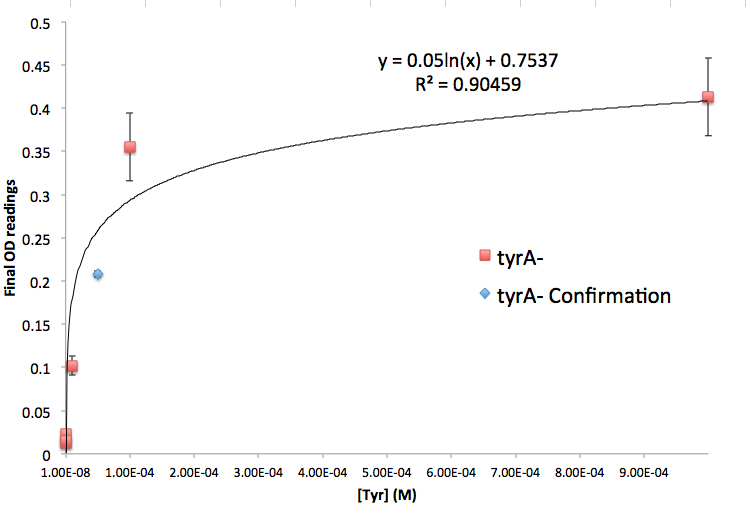
Also, we assumed that the amino acid consumption is solely depended on the cell growth and not the cell self maintenance. thus they are also obtained directly using our own wet lab data for these specific E. coli. We grew TrpA-, MetA-, and TryA- E. coli knock outs in 50 ml flasks with known initial Tryptophan, Methionine, and Tyrosine concentrations (5E-5 M) respectively, and harvested 5ml of cell free supernatants at various ODs. All these supernatants served as new 96-well plate media for each knock out cultures with newly added M9-Glucose shots (2% glucose in the well), which made sure the Amino Acid will be the limiting substrate for all knock outs. (Through previous experiments, we found that the each of the knockout monocultures will reach to a certain final OD, with respect to the media amino acid concentrations, thus we generated a calibration curve to relate the limiting amino acid concentration to their final OD readings.) These calibration curves are shown below:
Note: We also tested the accuracy of one of the calibration curves. The blue dot in the Figure#?? is another data value we obtained later at a known Tyrosine concentration (5E-5 M), and it is reasonably close to the predicted curve though not exactly the same. We are aware that this curve has limitation, mainly due to the big error bars at the higher OD data points, though this test point has proven that this calibration curve can serve us well at this stage of the research. Also, A Programmable Escherichia coli Consortium via Tunable Symbiosis also proved that this technique could be used for this purpose. (How should I say this, or should i say this at all??????????)
After growing the inoculated 96-well plate in humidified temperature control (37 deg) plate reader for 15 hours, their growth curves had been captured and most of the monocultures had reached their stationary phase, and the plate was then transferred to 37 deg room to obtain the remaining final OD readings.
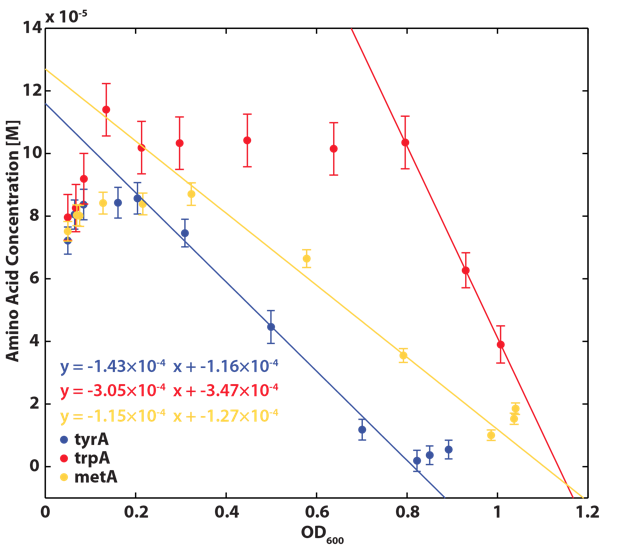
Based on the calibration curves above, the amino acid concentrations in the wells were calculated, and when corrected with a dilution factor of 5/4 (when we added in the M9-Glucose shot, it was 1/5 of the total well media volume), we arrived at the corresponding supernatant amino acid concentration at various monoculture OD readings. With the assumption that the only change in the amino acid concentration in the media is due to cell growths, and as they are specific knock outs, they were not able to produce any amino acid into the environment, each auxotrophs' consumption constant with respect to cell growth rate are calculated. Their values are:
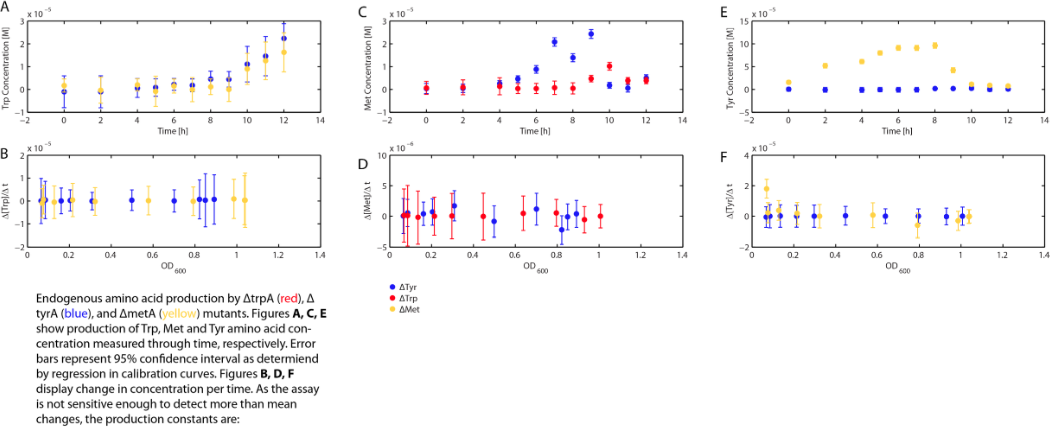
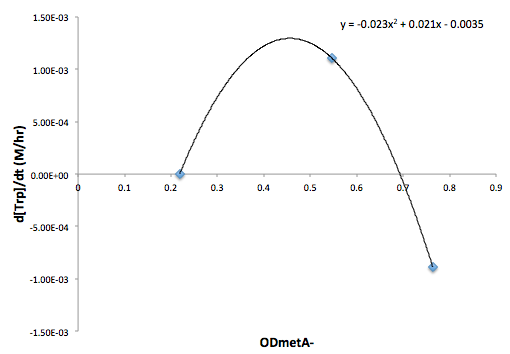
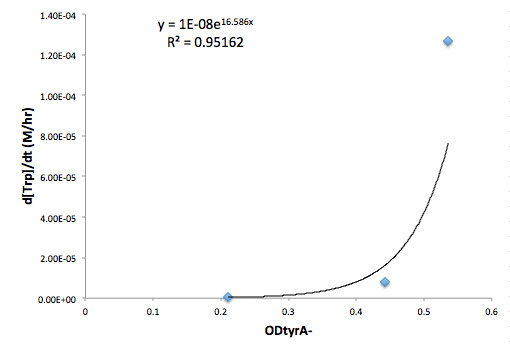
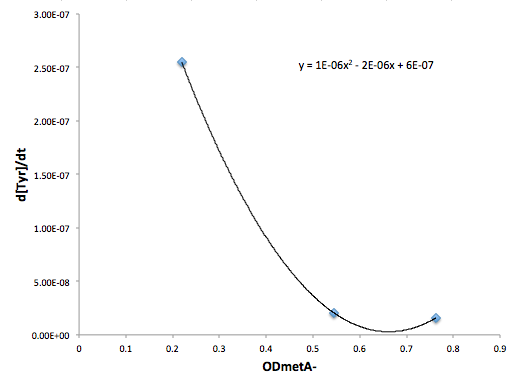
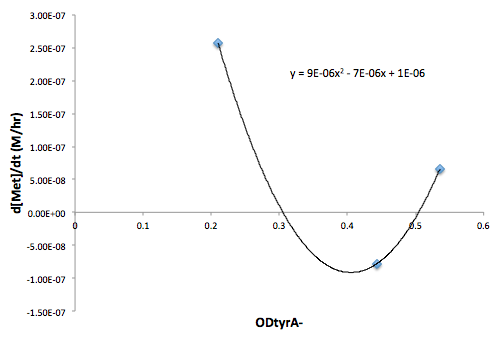
Moreover, in co-culture environment, to relate the contributions of the amino acid concentrations to the environment (media) of monocultures, the closest assumption we can make is that they will behave similarly to when they are in the monoculture conditions. Thus, we used the same method of relating the final OD to the limiting substrate amino acid concentration in the media, and found out the other two non-limiting amino acid concentrations of each knocked out E. coli auxotrophs (ΔtrpA, ΔmetA, and ΔtyrA) at various OD readings [2??]. With the consideration of sample taken time and some calculations, we were able to derive six functions that relate the change of all amino acid concentrations with respect to time (d[AA]/dt) with each auxotrophs' optical densities (ODaa), which is shown below:


Based on these approximated functions, we were able to obtain the equations used in equations (1), (2), and (3).
.
Matlab Code
Now with all the key functions and constants defined, we are able to encode our model into Matlab, where the six key ODE functions can be solved and output our prediction of population dynamics
The code is shown below:
and when run the program, it predicts the population dynamics without inducing as shown in the following diagram:
As mentioned at the beginning, this code can be generalized to predict the population dynamics when there is a tuning effect, therefore we can use this model to see decide the amount of tuning we need to control the desired relative population of each auxotrophs.
References
[1] Shuler, ML; Kargi, F; Bioprocess Engineering Basic Concepts, 2002 2nd edition. Prentice Hall PTR.
[2] ????The Consortia Paper (this will be cited many places, and should be consistent in format.
 "
"