Contents[hide] |
The Problem
Producing complex therapeutic proteins often requires biosynthesis in mammalian cells. This method of expressing proteins presents many obstacles. Our project focuses on one: getting a mammalian cell to express the desired protein when we want it to. Currently, bioreactors in industry rely heavily on small signaling molecules to get the cells to respond. The time it takes for these molecules to diffuse through the cell culture medium and the subsequent removal they require when the product is purified are huge problems. To allow a finer control over gene expression our project studied the implementation of two light-induced genetic (or optogenetic) switches. Both allow a tight regulation of gene expression and eliminate the problem of removing signaling molecules during the final purification of the protein that was synthesized.
Many of the proteins synthesized for therapeutic purposes can also be toxic to the cells that produce them. A fine control of gene expression in these cases is crucial to the viability of the cells being cultivated. Small signaling molecules aren't ideal for these purposes but light is instantaneous and easy to vary.
The Simple Switch
The first switch we studied is an untested fusion protein designed to act as a light-induced transcriptional activator in mamallian cells. The LovTAP-VP16 protein consists of a Lov2 domain (from ) a Trp repressor (from ) and a VP16 transactional activating domain (from ). A first version of this fusion protein was developed at the university of Chicago and later characterized and used as a bacterial repressor by the EPFL 2009 iGEM. The protein binds to a TRP promoter after undergoing a conformational change when exposed to light. By fusing the Lov-TAP protein to a viral promoter, the idea was to turn the protein into a light activated DNA binding protein. When the TRP promoter is bound to the LovTAP-VP16 protein the viral promoter on the C terminal recruits RNA polymerase II to the site and favors the transcription of the reporter gene next to the TRP promoter.
This pathway is simple and light activation of LovTAP-VP16 results in direct activation of transcription. There are also major obstacles this approach presents though. The protein needs to be localized in the nucleus, bind well to DNA and long enough to activate transcription.
For this system we will be using a DsRed readout to characterize the speed and efficiency of transcription by quantifying fluorescence.
The Complex Switch
In addition to LovTAP switch, we will be realizing another, more complex, melanopsin-based light switch developed by Fussennegger et al. In this switch, a light-sensitive membrane bound protein, melanopsin, is inserted to trigger a signalling cascade. The melanopsin opens calcium channels in the cell which activates the NFAT pathway. By inserting a readout gene next to an NFAT promoter, we can promote its expression by triggering the release of calcium with light. In our expriments we used GFP as our readout protein for the same reasons DsRed was used in the LovTAP-VP16 experiments. This optogenetic switch takes advantage of an already existing mammallian pathway and limits the potential flaws related to promoting transcription once the pathway is activated. However, calcium is a broad effector and can have unintended consequences. Different cell types also react differently to calcium influx and this approach might not be generalizable. Fussenegger's team were successful promoting expression in HEK (human embryo kidney) cells, and we will also try to get the pathway to work in CHO cells and compare the results to the work done in HEK cells.
Constructs
- Phy42 : PhCMV-melanopsin-pASV40
This vector was provided to us by Martin Fussenegger. It contains human melanopsin with a CMV promoter and antibiotic resistance for neomycin.
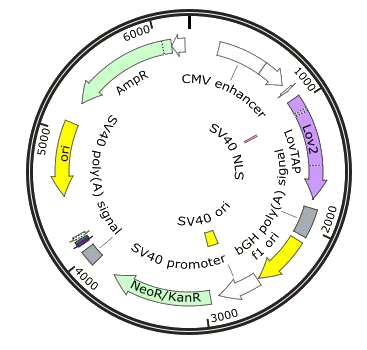
- Lov-Tap expression vector: PcDNA3.1 (+) - LovTap-VP16
LovTap-Vp16 was inserted into the MCS in front of a CMV promoter and followed by a poly A tail. The vector also contains resistance to hygromycin.
[http://products.invitrogen.com/ivgn/product/V79020 pcDNA3.1 + ]
- NFAT response vector: pGL4.30 - EGFP
We cloned in EGFP to replace luciferase in the original vector. This vector contains contains EGFP in front of an NFAT promoter and resistance to hygromycin.
[http://www.promega.com/resources/protocols/product-information-sheets/a/pgl430-vector-protocol/ pgl4.3]
- LovTap readout vector: Pcep4-TRP-DsRead
A TRP promoter and DsRed were cloned into the MCS. The constitutive promoter was also removed. This plasmid has hygromycin resistance.
[ http://products.invitrogen.com/ivgn/product/V04450 Pcep4]
Proteins
LovTAP-VP16
LovTAP-VP16 is the name of the protein our light switch is based on. But it has never been expresed before and, therefore, there is no literature telling us under what conditions it works or whether it works at all. It's a fusion protein between LovTAP, which is itself a fusion protein, and an activation domain from VP16, a viral transcription factor.
In this case we don't want any kind of steric interaction between LovTAP and VP16, since that might alter the functionality of one or both parts and we just want VP16 to be transported by LovTAP. To ensure this, a linker might be needed to physically separate the domains. Since LovTAP-VP16 has to be in the nucleus of the cell to work, the minimal linker would be a 7 residues long Nuclear Localazation Signal (NLS) from the virus SV40, with the amino aced sequece PKKKRKV.
In order to visually verify if a longer linker would be needed, we put together the existing crystal structures of the domains LovTAP-VP16 consists of: LOV2, TrpR, NLS and VP16.
LovTAP
In a paper published in 2008 [http://www.pnas.org/content/105/31/10709.abstract], Strickland et al. propose to modify the protein TrpR such that its activity can be controlled by light. This is done by fusing it to a light sensitive protein, the plant phototropin LOV2 (Light-Oxygen-Voltage), whose sensitivity to blue light is conferred by the ligand chromophore flavin mononucleotide (FMN). The fusion is done in a way that both domains share a common α-helix, which would create a sort of lever that could transfer the conformational changes in LOV2 when light-activated towards TrpR, triggering its activation.
Allosteric regulation
In a protein, generally an enzyme, an allosteric site is any part of the protein other than the active site.
Allosteric regulation of a protein consists in modifying its properties by interacting with an allosteric site. One example would be the regulation in the tryptophan (trp) operon, a group of genes studied in E. coli that are required for the synthesis of the amino acid tryptophan. The expression of these genes can be blocked by the homodimeric protein tryptophan repressor (TrpR), by binding the operator of the operon. TrpR repressing function is only active when tryptophan is bound to its allosteric sites, i.e. it blocks the production of tryptophan when the concentration of tryptophan is high.
TrpR
Dimerizes and bind the TrpO sequence: CGTACTAGTTAACTAGTACG
LOV2
Saturates at 20 mW/cm-2 irradiance at 470 nm according to Strickland 2008.
LOV2 photoproduct from phot1 has a half life of 30-40 s in Arabidopsis and rize. LovTAP has LOV2 from phot1 in Avena sativa. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC161699/table/TII/]
VP16
[http://pubs.acs.org/doi/full/10.1021/bi0482912|Paper] stating that the part of VP16 we used (456-490) behaves as transcriptional activator.
Future Work
Characterizing and troubleshooting the activating characteristics of the LovTAP-VP16 protein should be a priority in getting the simple switch system to work. It's still not clear whether or not it is localized in the nucleus and properly binds DNA with the additional VP16 promoter domain.
A nuclear exctract with western blotting or immunofluorescence techniques could be used to localise the protein. If the localisation of the protein isn't correct the nuclear localisation signal might need modification.
A Chromatin immunoprecipitation assay could be used to verify the binding characteristics of th LovTAP protein in the case where it is localised in the nucleus but still doesnt work.
Lastly, if the protein can bind DNA and is localized in the nuclues, a different promoter/linker combination could be used instead of VP16 to initiate transcription.
Other verions of LovTAP which respond better to light have also been characterized by Strickland et al.
[http://www.nature.com/nmeth/journal/v7/n8/full/nmeth.1473.html Improvement of Lov domain based photoswitches]
By using an improved version of the protein with a more dynamic response to light we could improve the DNA binding response to light and activation of trancsription.
Lastly, the creation of stable cell lines for each of our expression systems would make sense for a longer term project. The original experiment conducted by Fussenneger et al. used antibiotic resistance to select cells that had well integrated the transfected DNA. With a homogenous population of cells it is easier to characterize the efficiency of the system being used.
With our transient expression experiment for both switches we had heterogenous populations of cells. The efficiency of our transfections were around 30% and each cell integrated our vectors at different places in their genomes. The constitutive levels of LovTAP-VP16 or melanopsin proteins as well as the accesibility of the readout and promoter on the genome varied within the population of transfected cells.
It could be that our proteins behave the way we would like them to in a few cells among the many that were transfect but that most of the time integration of the transfected DNA is poor and expression levels are too low.
 "
"