Team:LMU-Munich/Bacillus BioBricks/vector use

The LMU-Munich team is exuberantly happy about the great success at the World Championship Jamboree in Boston. Our project Beadzillus finished 4th and won the prize for the "Best Wiki" (with Slovenia) and "Best New Application Project".
[ more news ]

How to work with Bacillus subtilis vectors
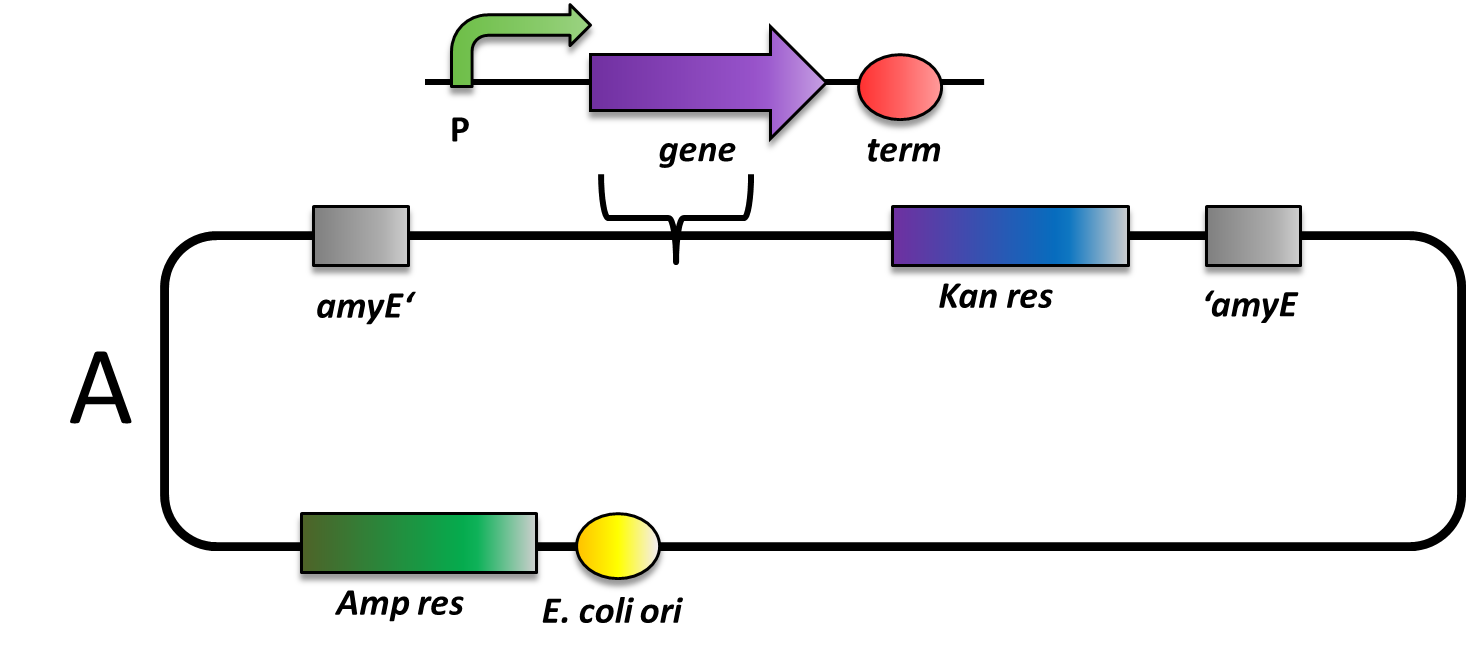
Many vectors for Bacillus subtilis are integrating into the genome, so do all but one that we provide to the registry.
There are some features in B. subtilis vectors that have to be taken into account, while working with them.
- Cloning in E.coli
- Linearisation before B. subtilis transformation
- Verification of integration
Pre-Cloning in E. coli
The cloning, that means the insertion of your part into the multiple cloning site of a vector, is done by normal ligations and E. coli. However, since the B. subtilis vectors are quite large, the cloning works best if only one insert is inserted. You could try to finish your construct in e.g. pSB1C3 and then clone it into the B. subtilis vector. For convenience, all vectors carry an RFP with promoter and terminator which is substituted by your insert during the ligation.
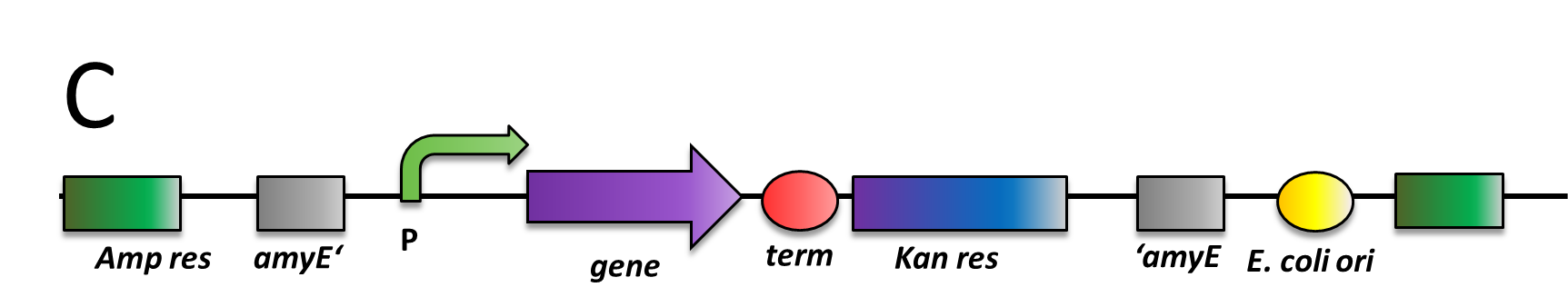
All our vectors carry the bla gene that mediates Ampicillin resistance (100 µg/ml) in E. coli. Also, they all have two recombination sites for integration into the B. subtilis genome. In between those recombination sites, there is the multiple cloning site and a resistance marker for B. subtilis.
(Some vectors from other working groups do not carry an extra E. coli resistance, so the B. subtilis resistance is also used in E. coli but with lower antibiotic concentrations. There is also the possibility of single-crossover plasmids which do also work fine, but the vector can then easily cross-out again, so it is not stably integrated.)
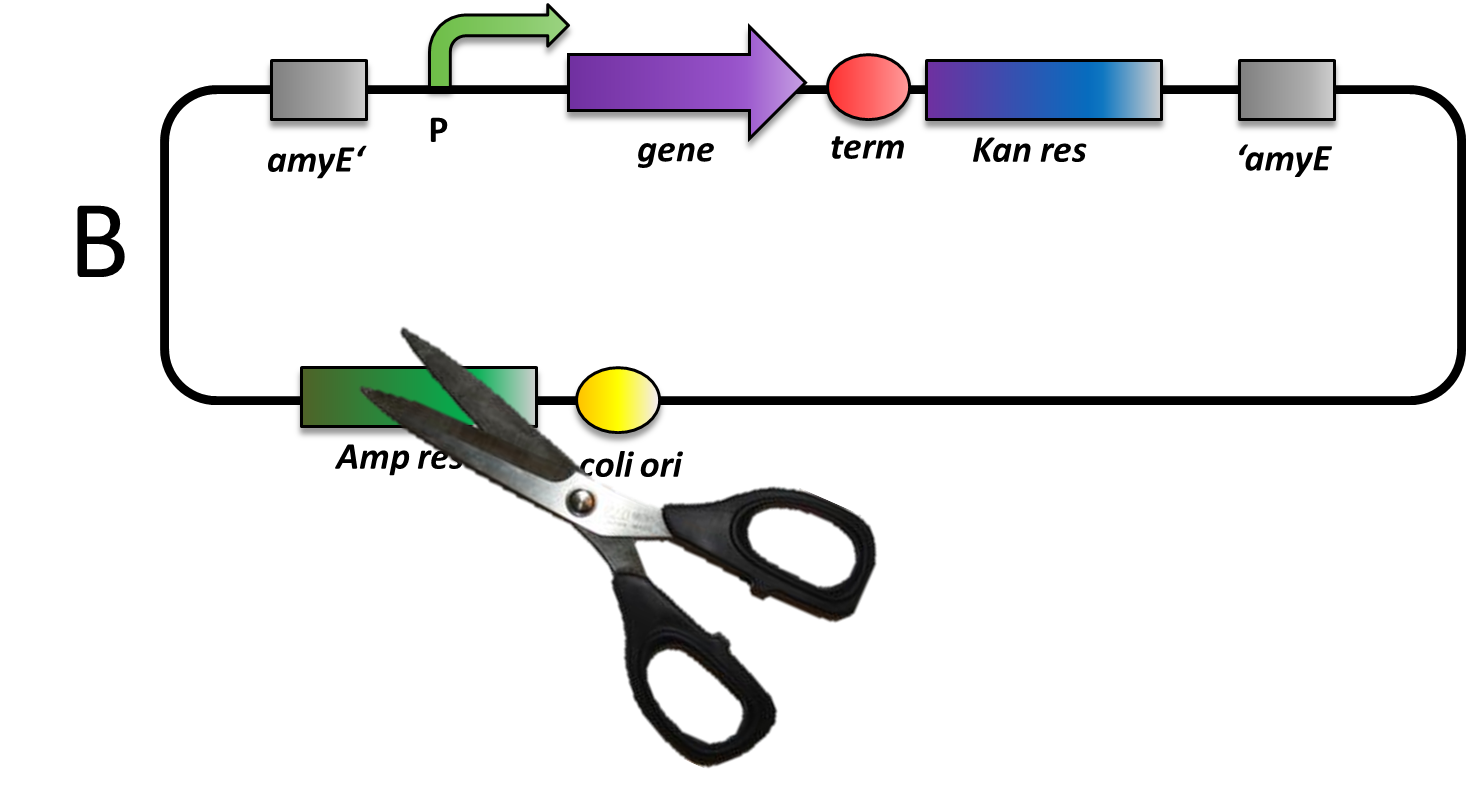
Linearisation before transformation in B. subtilis
For integration ofs by double crossover, plasmids (if it is not replicative) have to be linearized before transformation. B. subtilis is naturally competent, takes up DNA fragments and integrates preferably linear fragment into its genome via double cross-over. The linearization of our plasmids can all be performed with ScaI which cuts only inside the bla gene. If that enzyme also cuts in your insert, please check for other single cutters outside of the area that is integrated into the B. subtilis genome. For the actual transformation of B. subtilis, please linearize 1-2 µg of your plasmid and then proceed with our transformation protocol.
Verification of correct integration
Transformants are plated on selective media containing the appropriate antibiotic (resistance gene in between recombination sites). The obtained colonies then are tested for their insertion into the correct locus. Usually it is sufficient to test 4-8 colonies.
To test the insertion into the
-
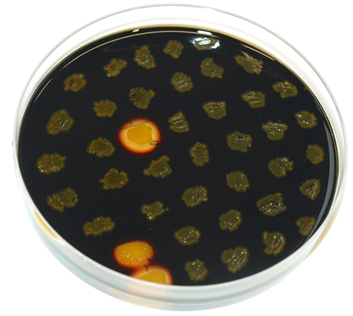
amyE-locus: amyE codes for an α-Amylase which degrades starch. Disruption of amyE disables B. subtilis of degrading starch. Starch is usually visualized by the starch-iodine reaction with Lugol's iodine that reveals a dark blue colour.
|
-
thrC-locus: thrC codes for the threonine synthase which performs an essential reaction for the production of the amino acid threonine. Disruption of that gene leads to threonine auxotrophy which can be tested for with minimal medium.Totest the transformants, streak the obtained colonies (and the WT as control) on a replica plate (with antibiotic) and on minimal medium without threonine and minimal medium with threonine (use the MNGE media, recipe see transformation protocol). Correct colonies should grow only on LB and minimal medium with threonine (see Figure 2).
|
-
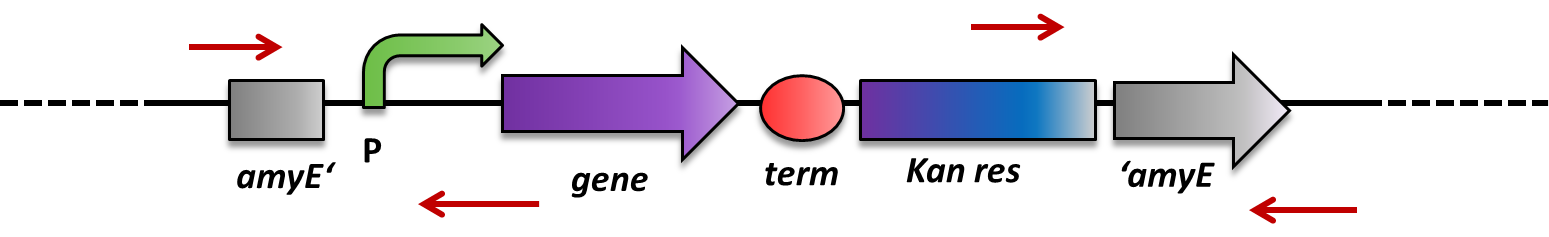
sacA-locus and lacA-locus: for those two loci, a colony PCR should be performed. The protocol can be found on our website. As shown below with an examplary locus, one of the primers of each pair is located on the genome, facing inwards, and the other one is located in between the recombination sites, facing outwards.
-
For sacA: you can use the following primers: up: TM2505: CTGATTGGCATGGCGATTGC together with TM2506: ACAGCTCCAGATCCTCTACG as well as down: TM2507: GTCGCTACCATTACCAGTTG together with TM2508: TCCAAACATTCCGGTGTTATC.
|
-
for lacA, you can use the primers: TM2624: GAACGAAGGGCTAAGAGAAC
and TM2625:AAGCAGAAGGCCATCCTGAC
(result: 650 bp)
as well as TM2624 and TM2627: AAGAATCCGCCCATATCGAG (result: 3000 bp+ length of construct)
With colony PCR you can check any other locus.
 "
"