Team:Wageningen UR/Journal/week25
From 2012.igem.org
Week 25: 15 october - 21 october
15 October
- 3rd time growing JM109 containing our biobricks BBa_K883702 (GFP coil with an IPTG induced promoter) and BBa_K883703 (GFP coil + His tag with an IPTG induced promoter) and induce expression with IPTG with new medium
-> no GFP production could be seen
16 October
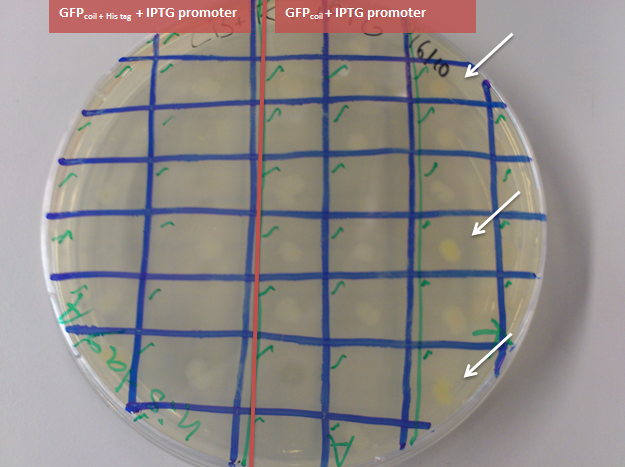
- to check if there are any colonies showing green fluorescence on the original plate (of the transformation on 21.September GFPcoil; GFPcoil + his tag in Bba_J04500 (IPTG induced promoter) in JM109), colonies where picked randomly and plated on agarplates containing IPTG next to the selection antibiotic
-> some colonies containing GFPcoil with an IPTG induced promoter show green fluorescence
- plate the JM109 samples that where used for sending the bricks in to the registry - containing BBa_K883702(GFPcoil with IPTG induced promoter) and BBa_K883703 (GFPcoil + His tag with IPTG induced promoter) on agarplates containing IPTG next to the selection antibiotic
-> culture containing BBa_K883702(GFPcoil with IPTG induced promoter) shows green fluorescence
- grow colonies containing the biobricks BBa_K883702 BBa_K883703 BBa_K883701 and BBa_K883700
- grow colonies containing BBa_K883702 and BBa_K883703 in duplo once with IPTG in the medium and once adding IPTG (fresh stock solution) to the culture at OD=0.6
- miniprep of the colonies containing BBa_K883702 BBa_K883703 BBa_K883701 and BBa_K883700
- 2nd try to send the bricks BBa_K883702 BBa_K883703 BBa_K883701 and BBa_K883700 in for sequencing
-> sequencing revealed that BBa_K883702 and BBa_K883703 had the expected sequence but BBa_K883701 and BBa_K883700 where faulty - therefore we deleted these two bricks from the registry
18 October
- transformation of BBa_K883702 with BL21 (producing strain) - plating the transformants on plates containing IPTG
19 October
- colony PCR of the transformation BBa_K883702 with BL21 using sequencing primers and plating the picked colonies again on a plate containing IPTG
-> all the colonies picked had the correct insert and showed fluorescence under the UV light
- transformation of BBa_K883703 (GFPcoil + his tag + IPTG promoter) with BL21 (producing strain)
- digestion of BBa_K883702 as well as BBa_K883703 and ligation with the pSB1C3 backbone (originating from BBa_J04450). These constructs where than transformed with DH5α and plated on plate containing IPTG

Figure 5: transformation of the ligation of BBa_K883702 with the pSB1C3 backbone in DH5α. The red colonies contain the original plasmid BBa_J04450 of the pSB1C3 backbone and encode for RFP with a constituate promoter, the green colonies contain the successful ligation product with the RFP encoding gene cut out and the GFPcoil + IPTG promoter ligated into the backbone (examples marked with an arrow). The circled ones are picked colonies'
Hepatitis B inside modification
15 October
- PCR check of the PCR fragment obtained on 27.August
- ligation of the PCR product in pJET
- transformation with DH5α
-> there was growth on the negative control plate
 "
"